Molecular aspects of transferrin expression in the tsetse fly (Glossina morsitans morsitans)
- PMID: 17498733
- PMCID: PMC2065764
- DOI: 10.1016/j.jinsphys.2007.03.013
Molecular aspects of transferrin expression in the tsetse fly (Glossina morsitans morsitans)
Abstract
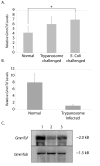
Iron is an essential element for metabolic processes intrinsic to life, and yet the properties that make iron a necessity also make it potentially deleterious. To avoid harm, iron homeostasis is achieved via proteins involved in transport and storage of iron, one of which is transferrin. We describe the temporal and spatial aspects of transferrin (GmmTsf) expression and its transcriptional regulation in tsetse where both the male and female are strictly hematophagous. Using Northern, Western and immunohistochemical analysis, we show that GmmTsf is abundant in the hemolymph and is expressed in the adult developmental stages of male and female insects. It is preferentially expressed in the female milk gland tubules and its expression appears to be cyclical and possibly regulated in synchrony with the oogenic and/or larvigenic cycle. Although no mRNA is detected, GmmTsf protein is present in the immature stages of development, apparently being transported into the intrauterine larva from the mother via the milk gland ducts. Transferrin is also detected in the vitellogenic ovary and the adult male testes, further supporting its classification as a vitellogenic protein. Similar to reports in other insects, transferrin mRNA levels increase upon bacterial challenge in tsetse suggesting that transferrin may play an additional role in immunity. Although transferrin expression is induced following bacterial challenge, it is significantly reduced in tsetse carrying midgut trypanosome infections. Analysis of tsetse that have cured the parasite challenge shows normal levels of GmmTsf. This observation suggests that the parasite in competing for the availability of limited dietary iron may manipulate host gene expression.
Figures
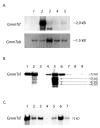
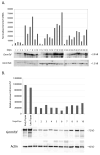
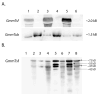
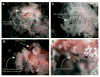
Similar articles
-
Molecular aspects of viviparous reproductive biology of the tsetse fly (Glossina morsitans morsitans): regulation of yolk and milk gland protein synthesis.J Insect Physiol. 2006 Nov-Dec;52(11-12):1128-36. doi: 10.1016/j.jinsphys.2006.07.007. Epub 2006 Sep 5. J Insect Physiol. 2006. PMID: 17046784 Free PMC article.
-
Differential expression of fat body genes in Glossina morsitans morsitans following infection with Trypanosoma brucei brucei.Int J Parasitol. 2008 Jan;38(1):93-101. doi: 10.1016/j.ijpara.2007.06.004. Epub 2007 Jul 1. Int J Parasitol. 2008. PMID: 17697681
-
Adult midgut expressed sequence tags from the tsetse fly Glossina morsitans morsitans and expression analysis of putative immune response genes.Genome Biol. 2003;4(10):R63. doi: 10.1186/gb-2003-4-10-r63. Epub 2003 Sep 11. Genome Biol. 2003. PMID: 14519198 Free PMC article.
-
Analysis of fat body transcriptome from the adult tsetse fly, Glossina morsitans morsitans.Insect Mol Biol. 2006 Aug;15(4):411-24. doi: 10.1111/j.1365-2583.2006.00649.x. Insect Mol Biol. 2006. PMID: 16907828
-
Computational characterization of Iron metabolism in the Tsetse disease vector, Glossina morsitans: IRE stem-loops.BMC Genomics. 2016 Aug 8;17:561. doi: 10.1186/s12864-016-2932-7. BMC Genomics. 2016. PMID: 27503259 Free PMC article.
Cited by
-
Sphingomyelinase activity in mother's milk is essential for juvenile development: a case from lactating tsetse flies.Biol Reprod. 2012 Jul 19;87(1):17, 1-10. doi: 10.1095/biolreprod.112.100008. Print 2012 Jul. Biol Reprod. 2012. PMID: 22517621 Free PMC article.
-
Molecular characterization of two novel milk proteins in the tsetse fly (Glossina morsitans morsitans).Insect Mol Biol. 2010 Apr;19(2):253-62. doi: 10.1111/j.1365-2583.2009.00987.x. Epub 2010 Feb 1. Insect Mol Biol. 2010. PMID: 20136662 Free PMC article.
-
Lipophorin acts as a shuttle of lipids to the milk gland during tsetse fly pregnancy.J Insect Physiol. 2011 Nov;57(11):1553-61. doi: 10.1016/j.jinsphys.2011.08.009. Epub 2011 Aug 22. J Insect Physiol. 2011. PMID: 21875592 Free PMC article.
-
Vitamin B6 generated by obligate symbionts is critical for maintaining proline homeostasis and fecundity in tsetse flies.Appl Environ Microbiol. 2014 Sep;80(18):5844-53. doi: 10.1128/AEM.01150-14. Epub 2014 Jul 18. Appl Environ Microbiol. 2014. PMID: 25038091 Free PMC article.
-
Gene expression in reproductive organs of tsetse females - initial data in an approach to reduce fecundity.BMC Microbiol. 2018 Nov 23;18(Suppl 1):144. doi: 10.1186/s12866-018-1294-5. BMC Microbiol. 2018. PMID: 30470199 Free PMC article.
References
-
- Aksoy S. Tsetse - A haven for microorganisms. Parasitology Today. 2000;16(3):114–118. - PubMed
-
- Attardo G, Strickler-Dinglasan P, Perkin S, Caler E, Bonaldo M, Soares M, El-Sayeed N, Aksoy S. Analysis of fat body transcriptome from the adult tsetse fly, Glossina morsitans morsitans. Insect Mol Biology. 2006a;15(4):411–424. - PubMed
-
- Barrett M. The fall and rise of sleeping sickness. Lancet. 1999;353:1113–1114. - PubMed
-
- Dunkov B, Georgieva T. Insect iron binding proteins: Insights from the genomes. Insect Biochemistry and Molecular Biology. 2006;36(4):300–309. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

