Deletion of PrBP/delta impedes transport of GRK1 and PDE6 catalytic subunits to photoreceptor outer segments
- PMID: 17496142
- PMCID: PMC1885592
- DOI: 10.1073/pnas.0701681104
Deletion of PrBP/delta impedes transport of GRK1 and PDE6 catalytic subunits to photoreceptor outer segments
Abstract
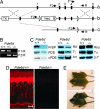
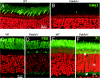
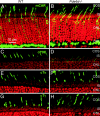
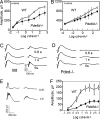
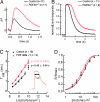
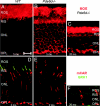
The mouse Pde6d gene encodes a ubiquitous prenyl binding protein, termed PrBP/delta, of largely unknown physiological function. PrBP/delta was originally identified as a putative rod cGMP phosphodiesterase (PDE6) subunit in the retina, where it is relatively abundant. To investigate the consequences of Pde6d deletion in retina, we generated a Pde6d(-/-) mouse by targeted recombination. Although manifesting reduced body weight, the Pde6d(-/-) mouse was viable and fertile and its retina developed normally. Immunocytochemistry showed that farnesylated rhodopsin kinase (GRK1) and prenylated rod PDE6 catalytic subunits partially mislocalized in Pde6d(-/-) rods, whereas rhodopsin was unaffected. In Pde6d(-/-) rod single-cell recordings, sensitivity to single photons was increased and saturating flash responses were prolonged. Pde6d(-/-) scotopic paired-flash electroretinograms indicated a delay in recovery of the dark state, likely due to reduced levels of GRK1 in rod outer segments. In Pde6d(-/-) cone outer segments, GRK1 and cone PDE6alpha' were present at very low levels and the photopic b-wave amplitudes were reduced by 70%. Thus the absence of PrBP/delta in retina impairs transport of prenylated proteins, particularly GRK1 and cone PDE, to rod and cone outer segments, resulting in altered photoreceptor physiology and a phenotype of a slowly progressing rod/cone dystrophy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Unc119 gene deletion partially rescues the GRK1 transport defect of Pde6d (- /-) cones.Adv Exp Med Biol. 2014;801:487-93. doi: 10.1007/978-1-4614-3209-8_62. Adv Exp Med Biol. 2014. PMID: 24664735 Free PMC article.
-
Mistrafficking of prenylated proteins causes retinitis pigmentosa 2.FASEB J. 2015 Mar;29(3):932-42. doi: 10.1096/fj.14-257915. Epub 2014 Nov 24. FASEB J. 2015. PMID: 25422369 Free PMC article.
-
A model for transport of membrane-associated phototransduction polypeptides in rod and cone photoreceptor inner segments.Vision Res. 2008 Feb;48(3):442-52. doi: 10.1016/j.visres.2007.08.020. Epub 2007 Oct 18. Vision Res. 2008. PMID: 17949773 Free PMC article. Review.
-
Evaluation of the 17-kDa prenyl-binding protein as a regulatory protein for phototransduction in retinal photoreceptors.J Biol Chem. 2005 Jan 14;280(2):1248-56. doi: 10.1074/jbc.M410475200. Epub 2004 Oct 25. J Biol Chem. 2005. PMID: 15504722 Free PMC article.
-
The prenyl-binding protein PrBP/δ: a chaperone participating in intracellular trafficking.Vision Res. 2012 Dec 15;75:19-25. doi: 10.1016/j.visres.2012.08.013. Epub 2012 Aug 29. Vision Res. 2012. PMID: 22960045 Free PMC article. Review.
Cited by
-
Expression and subcellular distribution of UNC119a, a protein partner of transducin α subunit in rod photoreceptors.Cell Signal. 2013 Jan;25(1):341-8. doi: 10.1016/j.cellsig.2012.10.005. Epub 2012 Oct 13. Cell Signal. 2013. PMID: 23072788 Free PMC article.
-
The interplay between RPGR, PDEδ and Arl2/3 regulate the ciliary targeting of farnesylated cargo.EMBO Rep. 2013 May;14(5):465-72. doi: 10.1038/embor.2013.37. Epub 2013 Apr 5. EMBO Rep. 2013. PMID: 23559067 Free PMC article.
-
Identification of a novel prenyl and palmitoyl modification at the CaaX motif of Cdc42 that regulates RhoGDI binding.Mol Cell Biol. 2013 Apr;33(7):1417-29. doi: 10.1128/MCB.01398-12. Epub 2013 Jan 28. Mol Cell Biol. 2013. PMID: 23358418 Free PMC article.
-
Cilia proteins getting to work - how do they commute from the cytoplasm to the base of cilia?J Cell Sci. 2022 Sep 1;135(17):jcs259444. doi: 10.1242/jcs.259444. Epub 2022 Sep 8. J Cell Sci. 2022. PMID: 36073764 Free PMC article. Review.
-
The small GTPase RAB28 is required for phagocytosis of cone outer segments by the murine retinal pigmented epithelium.J Biol Chem. 2018 Nov 9;293(45):17546-17558. doi: 10.1074/jbc.RA118.005484. Epub 2018 Sep 18. J Biol Chem. 2018. PMID: 30228185 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

