Small interfering RNA profiling reveals key role of clathrin-mediated endocytosis and early endosome formation for infection by respiratory syncytial virus
- PMID: 17494077
- PMCID: PMC1933373
- DOI: 10.1128/JVI.02780-06
Small interfering RNA profiling reveals key role of clathrin-mediated endocytosis and early endosome formation for infection by respiratory syncytial virus
Abstract
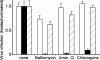
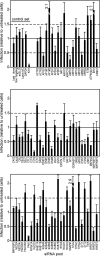
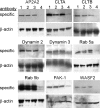
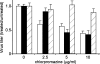
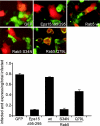
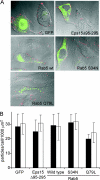
Respiratory syncytial virus (RSV) is a common cause of respiratory tract infections in infants and the elderly. Like many other pH-independent enveloped viruses, RSV is thought to enter at the cell surface, independently of common endocytic pathways. We have used a targeted small interfering RNA (siRNA) library to identify key cellular genes involved in cytoskeletal dynamics and endosome trafficking that are important for RSV infection. Surprisingly, RSV infection was potently inhibited by siRNAs targeting genes associated with clathrin-mediated endocytosis, including clathrin light chain. The important role of clathrin-mediated endocytosis was confirmed by the expression of well-characterized dominant-negative mutants of genes in this pathway and by using the clathrin endocytosis inhibitor chlorpromazine. We conclude that, while RSV may be competent to enter at the cell surface, clathrin function and endocytosis are a necessary and important part of a productive RSV infection, even though infection is strictly independent of pH. These findings raise the possibility that other pH-independent viruses may share a similar dependence on endocytosis for infection and provide a new potential avenue for treatment of infection.
Figures

Similar articles
-
The essential role of clathrin-mediated endocytosis in the infectious entry of human enterovirus 71.J Biol Chem. 2011 Jan 7;286(1):309-21. doi: 10.1074/jbc.M110.168468. Epub 2010 Oct 18. J Biol Chem. 2011. PMID: 20956521 Free PMC article.
-
Small interference RNA profiling reveals the essential role of human membrane trafficking genes in mediating the infectious entry of dengue virus.Virol J. 2010 Feb 1;7:24. doi: 10.1186/1743-422X-7-24. Virol J. 2010. PMID: 20122152 Free PMC article.
-
Role of clathrin-mediated endocytosis during vesicular stomatitis virus entry into host cells.Virology. 2005 Jul 20;338(1):53-60. doi: 10.1016/j.virol.2005.05.006. Virology. 2005. PMID: 15936793
-
Respiratory syncytial virus glycoproteins uptake occurs through clathrin-mediated endocytosis in a human epithelial cell line.Virol J. 2008 Oct 25;5:127. doi: 10.1186/1743-422X-5-127. Virol J. 2008. PMID: 18950517 Free PMC article. Review.
-
Respiratory syncytial virus persistence: evidence in the mouse model.Pediatr Infect Dis J. 2008 Oct;27(10 Suppl):S60-2. doi: 10.1097/INF.0b013e3181684d52. Pediatr Infect Dis J. 2008. PMID: 18820580 Review.
Cited by
-
Unity in diversity: shared mechanism of entry among paramyxoviruses.Prog Mol Biol Transl Sci. 2015;129:1-32. doi: 10.1016/bs.pmbts.2014.10.001. Epub 2014 Dec 1. Prog Mol Biol Transl Sci. 2015. PMID: 25595799 Free PMC article. Review.
-
Identification of host factors involved in borna disease virus cell entry through a small interfering RNA functional genetic screen.J Virol. 2010 Apr;84(7):3562-75. doi: 10.1128/JVI.02274-09. Epub 2010 Jan 13. J Virol. 2010. PMID: 20071576 Free PMC article.
-
The essential role of clathrin-mediated endocytosis in the infectious entry of human enterovirus 71.J Biol Chem. 2011 Jan 7;286(1):309-21. doi: 10.1074/jbc.M110.168468. Epub 2010 Oct 18. J Biol Chem. 2011. PMID: 20956521 Free PMC article.
-
Five Residues in the Apical Loop of the Respiratory Syncytial Virus Fusion Protein F2 Subunit Are Critical for Its Fusion Activity.J Virol. 2018 Jul 17;92(15):e00621-18. doi: 10.1128/JVI.00621-18. Print 2018 Aug 1. J Virol. 2018. PMID: 29743373 Free PMC article.
-
Roles of Cholesterol in Early and Late Steps of the Nipah Virus Membrane Fusion Cascade.J Virol. 2021 Feb 24;95(6):e02323-20. doi: 10.1128/JVI.02323-20. Print 2021 Feb 24. J Virol. 2021. PMID: 33408170 Free PMC article.
References
-
- Balachandran, S., P. C. Roberts, L. E. Brown, H. Truong, A. K. Pattnaik, D. R. Archer, and G. N. Barber. 2000. Essential role for the dsRNA-dependent protein kinase PKR in innate immunity to viral infection. Immunity 13:129-141. - PubMed
-
- Barnes, W. G., E. Reiter, J. D. Violin, X. R. Ren, G. Milligan, and R. J. Lefkowitz. 2005. β-Arrestin 1 and Gαq/11 coordinately activate RhoA and stress fiber formation following receptor stimulation. J. Biol. Chem. 280:8041-8050. - PubMed
-
- Benmerah, A., M. Bayrou, N. Cerf-Bensussan, and A. Dautry-Varsat. 1999. Inhibition of clathrin-coated pit assembly by an Eps15 mutant. J. Cell Sci. 112:1303-1311. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

