Role of mitochondrial remodeling in programmed cell death in Drosophila melanogaster
- PMID: 17488630
- PMCID: PMC1885957
- DOI: 10.1016/j.devcel.2007.02.002
Role of mitochondrial remodeling in programmed cell death in Drosophila melanogaster
Abstract
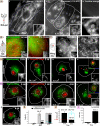
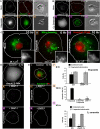
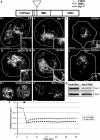
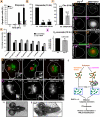
The role of mitochondria in Drosophila programmed cell death remains unclear, although certain gene products that regulate cell death seem to be evolutionarily conserved. We find that developmental programmed cell death stimuli in vivo and multiple apoptotic stimuli ex vivo induce dramatic mitochondrial fragmentation upstream of effector caspase activation, phosphatidylserine exposure, and nuclear condensation in Drosophila cells. Unlike genotoxic stress, a lipid cell death mediator induced an increase in mitochondrial contiguity prior to fragmentation of the mitochondria. Using genetic mutants and RNAi-mediated knockdown of drp-1, we find that Drp-1 not only regulates mitochondrial fission in normal cells, but mediates mitochondrial fragmentation during programmed cell death. Mitochondria in drp-1 mutants fail to fragment, resulting in hyperplasia of tissues in vivo and protection of cells from multiple apoptotic stimuli ex vivo. Thus, mitochondrial remodeling is capable of modifying the propensity of cells to undergo death in Drosophila.
Figures
Similar articles
-
The Ral/exocyst effector complex counters c-Jun N-terminal kinase-dependent apoptosis in Drosophila melanogaster.Mol Cell Biol. 2006 Dec;26(23):8953-63. doi: 10.1128/MCB.00506-06. Epub 2006 Sep 25. Mol Cell Biol. 2006. PMID: 17000765 Free PMC article.
-
Identification of a novel toxicophore in anti-cancer chemotherapeutics that targets mitochondrial respiratory complex I.Elife. 2020 May 20;9:e55845. doi: 10.7554/eLife.55845. Elife. 2020. PMID: 32432547 Free PMC article.
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Expression of Pseudomonas aeruginosa toxin ExoS effectively induces apoptosis in host cells.Infect Immun. 2006 Dec;74(12):6557-70. doi: 10.1128/IAI.00591-06. Epub 2006 Sep 11. Infect Immun. 2006. PMID: 16966406 Free PMC article.
-
Programmed death in bacteria.Microbiol Mol Biol Rev. 2000 Sep;64(3):503-14. doi: 10.1128/MMBR.64.3.503-514.2000. Microbiol Mol Biol Rev. 2000. PMID: 10974124 Free PMC article. Review.
Cited by
-
Control of mitochondrial structure and function by the Yorkie/YAP oncogenic pathway.Genes Dev. 2012 Sep 15;26(18):2027-37. doi: 10.1101/gad.183061.111. Epub 2012 Aug 27. Genes Dev. 2012. PMID: 22925885 Free PMC article.
-
Effect of ascorbic acid on cellular respiration with mitochondrial reductase in gingival fibroblast.Bioinformation. 2023 May 31;19(5):552-555. doi: 10.6026/97320630019552. eCollection 2023. Bioinformation. 2023. PMID: 37886148 Free PMC article.
-
Loss of dE2F compromises mitochondrial function.Dev Cell. 2013 Nov 25;27(4):438-51. doi: 10.1016/j.devcel.2013.10.002. Dev Cell. 2013. PMID: 24286825 Free PMC article.
-
New insights into mitochondrial structure during cell death.Exp Neurol. 2009 Aug;218(2):183-92. doi: 10.1016/j.expneurol.2009.05.021. Epub 2009 May 21. Exp Neurol. 2009. PMID: 19464290 Free PMC article. Review.
-
Programmed cell death acts at different stages of Drosophila neurodevelopment to shape the central nervous system.FEBS Lett. 2016 Aug;590(15):2435-2453. doi: 10.1002/1873-3468.12298. Epub 2016 Jul 28. FEBS Lett. 2016. PMID: 27404003 Free PMC article. Review.
References
-
- Adams J.M., Cory S. Apoptosomes: engines for caspase activation. Curr. Opin. Cell Biol. 2002;14:715–720. - PubMed
-
- Arama E., Agapite J., Steller H. Caspase activity and a specific cytochrome C are required for sperm differentiation in Drosophila. Dev. Cell. 2003;4:687–697. - PubMed
-
- Brand A.H., Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. - PubMed
-
- Chew S.K., Akdemir F., Chen P., Lu W.J., Mills K., Daish T., Kumar S., Rodriguez A., Abrams J.M. The apical caspase dronc governs programmed and unprogrammed cell death in Drosophila. Dev. Cell. 2004;7:897–907. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

