Functional and structural studies of the vaccinia virus virulence factor N1 reveal a Bcl-2-like anti-apoptotic protein
- PMID: 17485524
- PMCID: PMC2885619
- DOI: 10.1099/vir.0.82772-0
Functional and structural studies of the vaccinia virus virulence factor N1 reveal a Bcl-2-like anti-apoptotic protein
Abstract
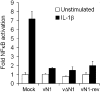
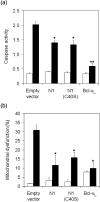
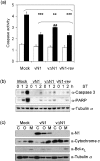
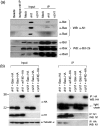
Vaccinia virus (VACV) encodes many immunomodulatory proteins, including inhibitors of apoptosis and modulators of innate immune signalling. VACV protein N1 is an intracellular homodimer that contributes to virus virulence and was reported to inhibit nuclear factor (NF)-kappaB signalling. However, analysis of NF-kappaB signalling in cells infected with recombinant viruses with or without the N1L gene showed no difference in NF-kappaB-dependent gene expression. Given that N1 promotes virus virulence, other possible functions of N1 were investigated and this revealed that N1 is an inhibitor of apoptosis in cells transfected with the N1L gene and in the context of VACV infection. In support of this finding virally expressed N1 co-precipitated with endogenous pro-apoptotic Bcl-2 proteins Bid, Bad and Bax as well as with Bad and Bax expressed by transfection. In addition, the crystal structure of N1 was solved to 2.9 A resolution (0.29 nm). Remarkably, although N1 shows no sequence similarity to cellular proteins, its three-dimensional structure closely resembles Bcl-x(L) and other members of the Bcl-2 protein family. The structure also reveals that N1 has a constitutively open surface groove similar to the grooves of other anti-apoptotic Bcl-2 proteins, which bind the BH3 motifs of pro-apoptotic Bcl-2 family members. Molecular modelling of BH3 peptides into the N1 surface groove, together with analysis of their physico-chemical properties, suggests a mechanism for the specificity of peptide recognition. This study illustrates the importance of the evolutionary conservation of structure, rather than sequence, in protein function and reveals a novel anti-apoptotic protein from orthopoxviruses.
Figures


Similar articles
-
Inhibition of apoptosis and NF-κB activation by vaccinia protein N1 occur via distinct binding surfaces and make different contributions to virulence.PLoS Pathog. 2011 Dec;7(12):e1002430. doi: 10.1371/journal.ppat.1002430. Epub 2011 Dec 15. PLoS Pathog. 2011. PMID: 22194685 Free PMC article.
-
Vaccinia virus proteins A52 and B14 Share a Bcl-2-like fold but have evolved to inhibit NF-kappaB rather than apoptosis.PLoS Pathog. 2008 Aug 15;4(8):e1000128. doi: 10.1371/journal.ppat.1000128. PLoS Pathog. 2008. PMID: 18704168 Free PMC article.
-
Vaccinia virus protein A49 is an unexpected member of the B-cell Lymphoma (Bcl)-2 protein family.J Biol Chem. 2015 Mar 6;290(10):5991-6002. doi: 10.1074/jbc.M114.624650. Epub 2015 Jan 20. J Biol Chem. 2015. PMID: 25605733 Free PMC article.
-
Structural biology of the Bcl-2 family of proteins.Biochim Biophys Acta. 2004 Mar 1;1644(2-3):83-94. doi: 10.1016/j.bbamcr.2003.08.012. Biochim Biophys Acta. 2004. PMID: 14996493 Review.
-
Bid: a Bax-like BH3 protein.Oncogene. 2008 Dec;27 Suppl 1:S93-104. doi: 10.1038/onc.2009.47. Oncogene. 2008. PMID: 19641510 Review.
Cited by
-
Increased ATP generation in the host cell is required for efficient vaccinia virus production.J Biomed Sci. 2009 Sep 2;16(1):80. doi: 10.1186/1423-0127-16-80. J Biomed Sci. 2009. PMID: 19725950 Free PMC article.
-
Poxvirus proteomics and virus-host protein interactions.Microbiol Mol Biol Rev. 2009 Dec;73(4):730-49. doi: 10.1128/MMBR.00026-09. Microbiol Mol Biol Rev. 2009. PMID: 19946139 Free PMC article. Review.
-
MHC-I-restricted epitopes conserved among variola and other related orthopoxviruses are recognized by T cells 30 years after vaccination.Arch Virol. 2008;153(10):1833-44. doi: 10.1007/s00705-008-0194-7. Epub 2008 Sep 12. Arch Virol. 2008. PMID: 18797815 Free PMC article.
-
Structural and biochemical bases for the inhibition of autophagy and apoptosis by viral BCL-2 of murine gamma-herpesvirus 68.PLoS Pathog. 2008 Feb 8;4(2):e25. doi: 10.1371/journal.ppat.0040025. PLoS Pathog. 2008. PMID: 18248095 Free PMC article.
-
A mechanism for the inhibition of DNA-PK-mediated DNA sensing by a virus.PLoS Pathog. 2013;9(10):e1003649. doi: 10.1371/journal.ppat.1003649. Epub 2013 Oct 3. PLoS Pathog. 2013. PMID: 24098118 Free PMC article.
References
-
- Bartlett, N., Symons, J. A., Tscharke, D. C. & Smith, G. L. (2002). The vaccinia virus N1L protein is an intracellular homodimer that promotes virulence. J Gen Virol 83, 1965–1976. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

