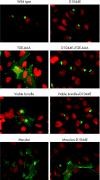
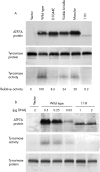
Phenotypic diversity of Menkes disease in mottled mice is associated with defects in localisation and trafficking of the ATP7A protein
- PMID: 17483305
- PMCID: PMC2597975
- DOI: 10.1136/jmg.2007.049627
Phenotypic diversity of Menkes disease in mottled mice is associated with defects in localisation and trafficking of the ATP7A protein
Abstract
Owing to mutations in the copper-transporting P-type ATPase, ATP7A (or MNK), patients with Menkes disease (MD) have an inadequate supply of copper to various copper-dependent enzymes. The ATP7A protein is located in the trans-Golgi network, where it transports copper via secretory compartments to copper-dependent enzymes. Raised copper concentrations result in the trafficking of ATP7A to the plasma membrane, where it functions in copper export. An important model of MD is the Mottled mouse, which possesses mutations in Atp7A. The Mottled mouse displays three distinct phenotypic severities: embryonic lethal, perinatal lethal and a longer-lived viable phenotype. However, the effects of mutations from these phenotypic classes on the ATP7A protein are unknown. In this study, we found that these classes of mutation differentially affect the copper transport and trafficking functions of the ATP7A protein. The embryonic lethal mutation, Atp7a(mo11H) (11H), caused mislocalisation of the protein to the endoplasmic reticulum, impaired glycosylation, and abolished copper delivery to the secretory pathway. In contrast, the perinatal lethal and viable mutations, Atp7a(moMac) (Macular) and Atp7a(moVbr) (Viable brindle) both resulted in a reduction in copper delivery to the secretory pathway and constitutive trafficking of the ATP7A protein to the plasma membrane in the absence of additional copper. In the case of Viable brindle, this hypertrafficking response was dependent on the catalytic phosphorylation site of ATP7A, whereas no such requirement was found for the Macular mutation. These findings provide evidence that the degree of MD severity in mice is associated with both copper transport and trafficking defects in the ATP7A protein.
Conflict of interest statement
Competing interests: None declared.
Similar articles
-
Mutation in the CPC motif-containing 6th transmembrane domain affects intracellular localization, trafficking and copper transport efficiency of ATP7A protein in mosaic mutant mice--an animal model of Menkes disease.Metallomics. 2012 Feb;4(2):197-204. doi: 10.1039/c1mt00134e. Epub 2011 Nov 16. Metallomics. 2012. PMID: 22089129
-
Small amounts of functional ATP7A protein permit mild phenotype.J Trace Elem Med Biol. 2015;31:173-7. doi: 10.1016/j.jtemb.2014.07.022. Epub 2014 Aug 8. J Trace Elem Med Biol. 2015. PMID: 25172213 Review.
-
Menkes disease.Cell Mol Life Sci. 2008 Jan;65(1):89-91. doi: 10.1007/s00018-007-7439-6. Cell Mol Life Sci. 2008. PMID: 17989919 Free PMC article. Review.
-
Alterations in the expression of the Atp7a gene in the early postnatal development of the mosaic mutant mice (Atp7a mo-ms) - An animal model for Menkes disease.Gene Expr Patterns. 2011 Jan-Feb;11(1-2):41-7. doi: 10.1016/j.gep.2010.09.001. Epub 2010 Sep 8. Gene Expr Patterns. 2011. PMID: 20831904
-
A conditional mutation affecting localization of the Menkes disease copper ATPase. Suppression by copper supplementation.J Biol Chem. 2002 Nov 15;277(46):44079-84. doi: 10.1074/jbc.M208737200. Epub 2002 Sep 6. J Biol Chem. 2002. PMID: 12221109
Cited by
-
Mutations in SLC33A1 cause a lethal autosomal-recessive disorder with congenital cataracts, hearing loss, and low serum copper and ceruloplasmin.Am J Hum Genet. 2012 Jan 13;90(1):61-8. doi: 10.1016/j.ajhg.2011.11.030. Am J Hum Genet. 2012. PMID: 22243965 Free PMC article.
-
The physiological and pathophysiological roles of copper in the nervous system.Eur J Neurosci. 2024 Jul;60(1):3505-3543. doi: 10.1111/ejn.16370. Epub 2024 May 15. Eur J Neurosci. 2024. PMID: 38747014 Review.
-
Investigation of iron metabolism in mice expressing a mutant Menke's copper transporting ATPase (Atp7a) protein with diminished activity (Brindled; Mo (Br) (/y) ).PLoS One. 2013 Jun 11;8(6):e66010. doi: 10.1371/journal.pone.0066010. Print 2013. PLoS One. 2013. PMID: 23776592 Free PMC article.
-
Altered intracellular localization and valosin-containing protein (p97 VCP) interaction underlie ATP7A-related distal motor neuropathy.Hum Mol Genet. 2012 Apr 15;21(8):1794-807. doi: 10.1093/hmg/ddr612. Epub 2011 Dec 30. Hum Mol Genet. 2012. PMID: 22210628 Free PMC article.
-
ATP7A Clinical Genetics Resource - A comprehensive clinically annotated database and resource for genetic variants in ATP7A gene.Comput Struct Biotechnol J. 2020 Sep 2;18:2347-2356. doi: 10.1016/j.csbj.2020.08.021. eCollection 2020. Comput Struct Biotechnol J. 2020. PMID: 32994893 Free PMC article.
References
-
- Prohaska J R, Gybina A A. Intracellular copper transport in mammals. J Nutr 20041341003–1006. - PubMed
-
- Kaler S G. Diagnosis and therapy of Menkes syndrome, a genetic form of copper deficiency. Am J Clin Nutr 1998671029–34S. - PubMed
-
- Monty J F, Llanos R M, Mercer J F, Kramer D R. Copper exposure induces trafficking of the menkes protein in intestinal epithelium of ATP7A transgenic mice. J Nutr 20051352762–2766. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases