Geranylgeranyltransferase I as a target for anti-cancer drugs
- PMID: 17476354
- PMCID: PMC1857249
- DOI: 10.1172/JCI32108
Geranylgeranyltransferase I as a target for anti-cancer drugs
Abstract
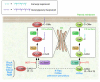
Posttranslational modification is critical for the function of the gene products of ras oncogenes, which are frequently mutated in cancer. Ras proteins are modified by farnesyltransferase (FTase), but many related small GTPases that also end in a CAAX motif (where C is cysteine, A is often an aliphatic amino acid, and X is any amino acid) are modified by a closely related enzyme known as geranylgeranyltransferase type I (GGTase-I). Accordingly, inhibitors for both of these enzymes have been developed, and those active against FTase are in clinical trials. In this issue of the JCI, Sjogren et al. report the development of a mouse strain homozygous for a conditional allele of the gene that encodes GGTase-I (see the related article beginning on page 1294). They found that ablation of the GGTase-I-encoding gene in cells destined to produce lung tumors driven by oncogenic K-Ras resulted in delayed onset and decreased severity of disease, validating in a genetic model the theory that GGTase-I is a good target for anti-cancer drug development.
Figures
Comment on
-
GGTase-I deficiency reduces tumor formation and improves survival in mice with K-RAS-induced lung cancer.J Clin Invest. 2007 May;117(5):1294-304. doi: 10.1172/JCI30868. J Clin Invest. 2007. PMID: 17476360 Free PMC article.
Similar articles
-
[Inhibitors of isoprenylation of ras p21].Gan To Kagaku Ryoho. 1997 Sep;24(11):1495-502. Gan To Kagaku Ryoho. 1997. PMID: 9309147 Review. Japanese.
-
Farnesyltransferase and geranylgeranyltransferase I inhibitors and cancer therapy: lessons from mechanism and bench-to-bedside translational studies.Oncogene. 2000 Dec 27;19(56):6584-93. doi: 10.1038/sj.onc.1204146. Oncogene. 2000. PMID: 11426643 Review.
-
GGTase-I deficiency reduces tumor formation and improves survival in mice with K-RAS-induced lung cancer.J Clin Invest. 2007 May;117(5):1294-304. doi: 10.1172/JCI30868. J Clin Invest. 2007. PMID: 17476360 Free PMC article.
-
Targeting the protein prenyltransferases efficiently reduces tumor development in mice with K-RAS-induced lung cancer.Proc Natl Acad Sci U S A. 2010 Apr 6;107(14):6471-6. doi: 10.1073/pnas.0908396107. Epub 2010 Mar 22. Proc Natl Acad Sci U S A. 2010. PMID: 20308544 Free PMC article.
-
Inactivation of farnesyltransferase and geranylgeranyltransferase I by caspase-3: cleavage of the common alpha subunit during apoptosis.Oncogene. 2001 Jan 18;20(3):358-66. doi: 10.1038/sj.onc.1204099. Oncogene. 2001. PMID: 11313965
Cited by
-
Dissecting the roles of DR4, DR5 and c-FLIP in the regulation of geranylgeranyltransferase I inhibition-mediated augmentation of TRAIL-induced apoptosis.Mol Cancer. 2010 Jan 29;9:23. doi: 10.1186/1476-4598-9-23. Mol Cancer. 2010. PMID: 20113484 Free PMC article.
-
Synthesis and screening of a CaaL peptide library versus FTase reveals a surprising number of substrates.Bioorg Med Chem Lett. 2010 Jan 15;20(2):767-70. doi: 10.1016/j.bmcl.2009.11.011. Epub 2009 Nov 12. Bioorg Med Chem Lett. 2010. PMID: 20005705 Free PMC article.
-
Exploring protein lipidation with chemical biology.Chem Rev. 2011 Oct 12;111(10):6341-58. doi: 10.1021/cr2001977. Epub 2011 Sep 16. Chem Rev. 2011. PMID: 21919527 Free PMC article. Review. No abstract available.
-
Geranylgeranyl diphosphate synthase inhibition induces apoptosis that is dependent upon GGPP depletion, ERK phosphorylation and caspase activation.Cell Death Dis. 2017 Mar 16;8(3):e2678. doi: 10.1038/cddis.2017.101. Cell Death Dis. 2017. PMID: 28300835 Free PMC article.
-
Effects of silencing farnesyltransferase on the migration, invasion, and epithelial-mesenchymal transition of salivary adenoid cystic carcinoma cells.Hua Xi Kou Qiang Yi Xue Za Zhi. 2022 Jul 25;40(4):394-402. doi: 10.7518/hxkq.2022.04.004. Hua Xi Kou Qiang Yi Xue Za Zhi. 2022. PMID: 38596954 Free PMC article. Chinese, English.
References
-
- Reiss Y., Goldstein J.L., Seabra M.C., Casey P.J., Brown M.S. Inhibition of purified p21ras farnesyl:protein transferase by Cys-AAX tetrapeptides. . Cell. 1990;62:81–88. - PubMed
-
- Seabra M.C., Reiss Y., Casey P.J., Brown M.S., Goldstein J.L. Protein farnesyltransferase and geranylgeranyltransferase share a common alpha subunit. Cell. 1991;65:429–434. - PubMed
-
- Whyte D.B., et al. K- and N-Ras are geranylgeranylated in cells treated with farnesyl protein transferase inhibitors. J. Biol. Chem. 1997;272:14459–14464. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

