LKB1 and AMPK maintain epithelial cell polarity under energetic stress
- PMID: 17470638
- PMCID: PMC2064817
- DOI: 10.1083/jcb.200702053
LKB1 and AMPK maintain epithelial cell polarity under energetic stress
Retraction in
-
LKB1 and AMPK maintain epithelial cell polarity under energetic stress.J Cell Biol. 2013 Oct 28;203(2):373. doi: 10.1083/jcb.20070205310112013r. J Cell Biol. 2013. PMID: 24165941 Free PMC article. No abstract available.
Abstract
LKB1 is mutated in both familial and spontaneous tumors, and acts as a master kinase that activates the PAR-1 polarity kinase and the adenosine 5'monophosphate-activated kinase (AMPK). This has led to the hypothesis that LKB1 acts as a tumor suppressor because it is required to maintain cell polarity and growth control through PAR-1 and AMPK, respectively. However, the genetic analysis of LKB1-AMPK signaling in vertebrates has been complicated by the existence of multiple redundant AMPK subunits. We describe the identification of mutations in the single Drosophila melanogaster AMPK catalytic subunit AMPKalpha. Surprisingly, ampkalpha mutant epithelial cells lose their polarity and overproliferate under energetic stress. LKB1 is required in vivo for AMPK activation, and lkb1 mutations cause similar energetic stress-dependent phenotypes to ampkalpha mutations. Furthermore, lkb1 phenotypes are rescued by a phosphomimetic version of AMPKalpha. Thus, LKB1 signals through AMPK to coordinate epithelial polarity and proliferation with cellular energy status, and this might underlie the tumor suppressor function of LKB1.
Figures
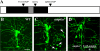
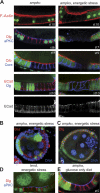
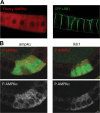
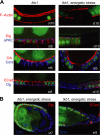

Similar articles
-
LKB1 regulates polarity remodeling and adherens junction formation in the Drosophila eye.Proc Natl Acad Sci U S A. 2009 Jun 2;106(22):8941-6. doi: 10.1073/pnas.0812469106. Epub 2009 May 14. Proc Natl Acad Sci U S A. 2009. PMID: 19443685 Free PMC article.
-
The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress.Proc Natl Acad Sci U S A. 2004 Mar 9;101(10):3329-35. doi: 10.1073/pnas.0308061100. Epub 2004 Feb 25. Proc Natl Acad Sci U S A. 2004. PMID: 14985505 Free PMC article.
-
Energy-dependent regulation of cell structure by AMP-activated protein kinase.Nature. 2007 Jun 21;447(7147):1017-20. doi: 10.1038/nature05828. Epub 2007 May 7. Nature. 2007. PMID: 17486097
-
AMPKα-like proteins as LKB1 downstream targets in cell physiology and cancer.J Mol Med (Berl). 2021 May;99(5):651-662. doi: 10.1007/s00109-021-02040-y. Epub 2021 Mar 4. J Mol Med (Berl). 2021. PMID: 33661342 Review.
-
Dialogue between LKB1 and AMPK: a hot topic at the cellular pole.Sci STKE. 2007 Sep 18;2007(404):pe51. doi: 10.1126/stke.4042007pe51. Sci STKE. 2007. PMID: 17878409 Review.
Cited by
-
Metabolic stress regulates cytoskeletal dynamics and metastasis of cancer cells.J Clin Invest. 2013 Jul;123(7):2907-20. doi: 10.1172/JCI67841. Epub 2013 Jun 10. J Clin Invest. 2013. PMID: 23921130 Free PMC article.
-
Cdx2 regulates endo-lysosomal function and epithelial cell polarity.Genes Dev. 2010 Jun 15;24(12):1295-305. doi: 10.1101/gad.1921510. Genes Dev. 2010. PMID: 20551175 Free PMC article.
-
The PAR proteins: fundamental players in animal cell polarization.Dev Cell. 2007 Nov;13(5):609-622. doi: 10.1016/j.devcel.2007.10.007. Dev Cell. 2007. PMID: 17981131 Free PMC article. Review.
-
AMP-activated protein kinase (AMPK) beyond metabolism: a novel genomic stress sensor participating in the DNA damage response pathway.Cancer Biol Ther. 2014 Feb;15(2):156-69. doi: 10.4161/cbt.26726. Epub 2013 Nov 1. Cancer Biol Ther. 2014. PMID: 24100703 Free PMC article. Review.
-
LKB1 regulates polarity remodeling and adherens junction formation in the Drosophila eye.Proc Natl Acad Sci U S A. 2009 Jun 2;106(22):8941-6. doi: 10.1073/pnas.0812469106. Epub 2009 May 14. Proc Natl Acad Sci U S A. 2009. PMID: 19443685 Free PMC article.
References
-
- Afonso, C., and D. Henrique. 2006. PAR3 acts as a molecular organizer to define the apical domain of chick neuroepithelial cells. J. Cell Sci. 119:4293–4304. - PubMed
-
- Alessi, D.R., K. Sakamoto, and J.R. Bayascas. 2006. Lkb1-dependent signaling pathways. Annu. Rev. Biochem. 75:137–163. - PubMed
-
- Baas, A.F., J. Kuipers, N.N. van der Wel, E. Batlle, H.K. Koerten, P.J. Peters, and H.C. Clevers. 2004. Complete polarization of single intestinal epithelial cells upon activation of LKB1 by STRAD. Cell. 116:457–466. - PubMed
-
- Benton, R., and D. St Johnston. 2003. Drosophila PAR-1 and 14-3-3 inhibit Bazooka/PAR-3 to establish complementary cortical domains in polarized cells. Cell. 115:691–704. - PubMed
-
- Bilder, D., and N. Perrimon. 2000. Localization of apical epithelial determinants by the basolateral PDZ protein Scribble. Nature. 403:676–680. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

