The emerging role of ACE2 in physiology and disease
- PMID: 17464936
- PMCID: PMC7167724
- DOI: 10.1002/path.2162
The emerging role of ACE2 in physiology and disease
Abstract
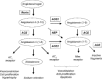
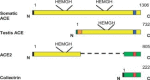
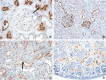
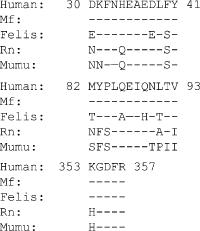
The renin-angiotensin-aldosterone system (RAAS) is a key regulator of systemic blood pressure and renal function and a key player in renal and cardiovascular disease. However, its (patho)physiological roles and its architecture are more complex than initially anticipated. Novel RAAS components that may add to our understanding have been discovered in recent years. In particular, the human homologue of ACE (ACE2) has added a higher level of complexity to the RAAS. In a short period of time, ACE2 has been cloned, purified, knocked-out, knocked-in; inhibitors have been developed; its 3D structure determined; and new functions have been identified. ACE2 is now implicated in cardiovascular and renal (patho)physiology, diabetes, pregnancy, lung disease and, remarkably, ACE2 serves as a receptor for SARS and NL63 coronaviruses. This review covers available information on the genetic, structural and functional properties of ACE2. Its role in a variety of (patho)physiological conditions and therapeutic options of modulation are discussed.
Copyright (c) 2007 Pathological Society of Great Britain and Ireland. Published by John Wiley & Sons, Ltd.
Figures

Similar articles
-
Angiotensin-converting enzyme 2 and new insights into the renin-angiotensin system.Biochem Pharmacol. 2008 Feb 15;75(4):781-6. doi: 10.1016/j.bcp.2007.08.012. Epub 2007 Aug 17. Biochem Pharmacol. 2008. PMID: 17897633 Free PMC article. Review.
-
Lessons from SARS: control of acute lung failure by the SARS receptor ACE2.J Mol Med (Berl). 2006 Oct;84(10):814-20. doi: 10.1007/s00109-006-0094-9. Epub 2006 Sep 19. J Mol Med (Berl). 2006. PMID: 16988814 Free PMC article. Review.
-
ACE2 (Angiotensin-Converting Enzyme 2) in Cardiopulmonary Diseases: Ramifications for the Control of SARS-CoV-2.Hypertension. 2020 Sep;76(3):651-661. doi: 10.1161/HYPERTENSIONAHA.120.15595. Epub 2020 Aug 12. Hypertension. 2020. PMID: 32783758 Free PMC article. Review.
-
Trilogy of ACE2: a peptidase in the renin-angiotensin system, a SARS receptor, and a partner for amino acid transporters.Pharmacol Ther. 2010 Oct;128(1):119-28. doi: 10.1016/j.pharmthera.2010.06.003. Epub 2010 Jul 3. Pharmacol Ther. 2010. PMID: 20599443 Free PMC article. Review.
-
Regulation of Angiotensin-Converting Enzyme 2: A Potential Target to Prevent COVID-19?Front Endocrinol (Lausanne). 2021 Oct 22;12:725967. doi: 10.3389/fendo.2021.725967. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34745001 Free PMC article. Review.
Cited by
-
Angiotensin-converting enzyme 2 receptors, chronic liver diseases, common medications, and clinical outcomes in coronavirus disease 2019 patients.World J Virol. 2021 May 25;10(3):86-96. doi: 10.5501/wjv.v10.i3.86. World J Virol. 2021. PMID: 34079691 Free PMC article. Review.
-
Role of Tissue Factor in the Pathogenesis of COVID-19 and the Possible Ways to Inhibit It.Clin Appl Thromb Hemost. 2021 Jan-Dec;27:10760296211003983. doi: 10.1177/10760296211003983. Clin Appl Thromb Hemost. 2021. PMID: 33784877 Free PMC article. Review.
-
Rapid Generation of Circulating and Mucosal Decoy Human ACE2 using mRNA Nanotherapeutics for the Potential Treatment of SARS-CoV-2.Adv Sci (Weinh). 2022 Dec;9(35):e2202556. doi: 10.1002/advs.202202556. Epub 2022 Oct 10. Adv Sci (Weinh). 2022. PMID: 36216580 Free PMC article.
-
Engineering mesenchymal stromal cells with neutralizing and anti-inflammatory capability against SARS-CoV-2 infection.Mol Ther Methods Clin Dev. 2021 Jun 11;21:754-764. doi: 10.1016/j.omtm.2021.05.004. Epub 2021 May 14. Mol Ther Methods Clin Dev. 2021. PMID: 34007862 Free PMC article.
-
Enhanced angiotensin converting enzyme 2 regulates the insulin/Akt signalling pathway by blockade of macrophage migration inhibitory factor expression.Br J Pharmacol. 2008 Jan;153(1):66-74. doi: 10.1038/sj.bjp.0707482. Epub 2007 Oct 1. Br J Pharmacol. 2008. PMID: 17906677 Free PMC article.
References
-
- Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, et al. A novel angiotensin‐converting enzyme‐related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ Res 2000; 87: E1–E9. - PubMed
-
- Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin‐converting enzyme. Cloning and functional expression as a captopril‐insensitive carboxypeptidase. J Biol Chem 2000; 275: 33238–33243. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

