Effects of methyl beta-cyclodextrin on EDHF responses in pig and rat arteries; association between SK(Ca) channels and caveolin-rich domains
- PMID: 17450174
- PMCID: PMC2013982
- DOI: 10.1038/sj.bjp.0707222
Effects of methyl beta-cyclodextrin on EDHF responses in pig and rat arteries; association between SK(Ca) channels and caveolin-rich domains
Abstract
Background and purpose: The small and intermediate conductance, Ca2+-sensitive K+ channels (SK(Ca) and IK(Ca), respectively) which are pivotal in the EDHF pathway may be differentially activated. The importance of caveolae in the functioning of IK(Ca) and SK(Ca) channels was investigated.
Experimental approach: The effect of the caveolae-disrupting agent methyl-beta-cyclodextrin (MbetaCD) on IK(Ca) and SK(Ca) localization and function was determined.
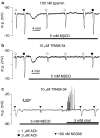
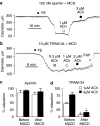
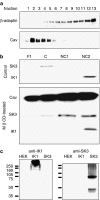
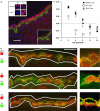
Key results: EDHF-mediated, SK(Ca)-dependent myocyte hyperpolarizations evoked by acetylcholine in rat mesenteric arteries (following blockade of IK(Ca) with TRAM-34) were inhibited by MbetaCD. Hyperpolarizations evoked by direct SK(Ca) channel activation (using NS309 in the presence of TRAM-34) were also inhibited by MbetaCD, an effect reversed by cholesterol. In contrast, IK(Ca)-dependent hyperpolarizations (in the presence of apamin) were unaffected by MbetaCD. Similarly, in porcine coronary arteries, EDHF-mediated, SK(Ca)-dependent (but not IK(Ca)-dependent) endothelial cell hyperpolarizations evoked by substance P were inhibited by MbetaCD. In mesenteric artery homogenates subjected to sucrose-density centrifugation, caveolin-1 and SK3 (SK(Ca)) proteins but not IK1 (IK(Ca)) protein migrated to the buoyant, caveolin-rich fraction. MbetaCD pretreatment redistributed caveolin-1 and SK3 proteins into more dense fractions. In immunofluorescence images of porcine coronary artery endothelium, SK3 (but not IK1) and caveolin-1 were co-localized. Furthermore, caveolin-1 immunoprecipitates prepared from native porcine coronary artery endothelium contained SK3 but not IK1 protein.
Conclusions and implications: These data provide strong evidence that endothelial cell SK(Ca) channels are located in caveolae while the IK(Ca) channels reside in a different membrane compartment. These studies reveal cellular organisation as a further complexity in the EDHF pathway signalling cascade.
Figures
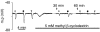

Similar articles
-
Opening of small and intermediate calcium-activated potassium channels induces relaxation mainly mediated by nitric-oxide release in large arteries and endothelium-derived hyperpolarizing factor in small arteries from rat.J Pharmacol Exp Ther. 2011 Dec;339(3):842-50. doi: 10.1124/jpet.111.179242. Epub 2011 Aug 31. J Pharmacol Exp Ther. 2011. PMID: 21880870
-
Characterization of an apamin-sensitive small-conductance Ca(2+)-activated K(+) channel in porcine coronary artery endothelium: relevance to EDHF.Br J Pharmacol. 2002 Mar;135(5):1133-43. doi: 10.1038/sj.bjp.0704551. Br J Pharmacol. 2002. PMID: 11877319 Free PMC article.
-
Role of SK(Ca) and IK(Ca) in endothelium-dependent hyperpolarizations of the guinea-pig isolated carotid artery.Br J Pharmacol. 2005 Feb;144(4):477-85. doi: 10.1038/sj.bjp.0706003. Br J Pharmacol. 2005. PMID: 15655533 Free PMC article.
-
Endothelium-derived hyperpolarising factors and associated pathways: a synopsis.Pflugers Arch. 2010 May;459(6):863-79. doi: 10.1007/s00424-010-0817-1. Epub 2010 Apr 11. Pflugers Arch. 2010. PMID: 20383718 Review.
-
Endothelial Ca+-activated K+ channels in normal and impaired EDHF-dilator responses--relevance to cardiovascular pathologies and drug discovery.Br J Pharmacol. 2009 Jun;157(4):509-26. doi: 10.1111/j.1476-5381.2009.00132.x. Epub 2009 Mar 19. Br J Pharmacol. 2009. PMID: 19302590 Free PMC article. Review.
Cited by
-
Eugenol dilates mesenteric arteries and reduces systemic BP by activating endothelial cell TRPV4 channels.Br J Pharmacol. 2015 Jul;172(14):3484-94. doi: 10.1111/bph.13156. Epub 2015 May 19. Br J Pharmacol. 2015. PMID: 25832173 Free PMC article.
-
Endothelium-Derived Hyperpolarization and Coronary Vasodilation: Diverse and Integrated Roles of Epoxyeicosatrienoic Acids, Hydrogen Peroxide, and Gap Junctions.Microcirculation. 2016 Jan;23(1):15-32. doi: 10.1111/micc.12255. Microcirculation. 2016. PMID: 26541094 Free PMC article. Review.
-
Vascular mechanotransduction.Physiol Rev. 2023 Apr 1;103(2):1247-1421. doi: 10.1152/physrev.00053.2021. Epub 2023 Jan 5. Physiol Rev. 2023. PMID: 36603156 Free PMC article. Review.
-
Aldosterone and vascular mineralocorticoid receptors: regulators of ion channels beyond the kidney.Hypertension. 2014 Apr;63(4):632-7. doi: 10.1161/HYPERTENSIONAHA.113.01273. Epub 2013 Dec 30. Hypertension. 2014. PMID: 24379184 Free PMC article. Review. No abstract available.
-
Inwardly rectifying K+ channels are major contributors to flow-induced vasodilatation in resistance arteries.J Physiol. 2017 Apr 1;595(7):2339-2364. doi: 10.1113/JP273255. Epub 2016 Dec 26. J Physiol. 2017. PMID: 27859264 Free PMC article.
References
-
- Burnham MP, Johnson IT, Weston AH. Reduced Ca2+-dependent activation of large-conductance Ca2+-activated K+ channels from arteries of Type 2 diabetic Zucker diabetic fatty rats. Am J Physiol. 2006b;290:H1520–H1527. - PubMed
-
- Busse R, Edwards G, Félétou M, Fleming I, Vanhoutte PM, Weston AH. EDHF: bringing the concepts together. Trends Pharmacol Sci. 2002;23:374–380. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

