Expression of tumor necrosis factor receptor-1 in arterial wall cells promotes atherosclerosis
- PMID: 17442899
- PMCID: PMC2522308
- DOI: 10.1161/ATVBAHA.0000261548.49790.63
Expression of tumor necrosis factor receptor-1 in arterial wall cells promotes atherosclerosis
Abstract
Objective: Mechanisms by which tumor necrosis factor-alpha (TNF) contributes to atherosclerosis remain largely obscure. We therefore sought to determine the role of the arterial wall TNF receptor-1 (TNFR1) in atherogenesis.
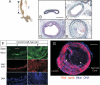
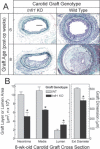
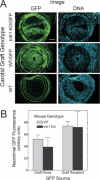
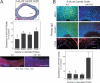
Methods and results: Carotid artery-to-carotid artery interposition grafting was performed with tnfr1-/- and congenic (C57Bl/6) wild-type (WT) mice as graft donors, and congenic chow-fed apolipoprotein E-deficient mice as recipients. Advanced atherosclerotic graft lesions developed within 8 weeks, and had 2-fold greater area in WT than in tnfr1-/- grafts. While the prevalence of specific atheroma cells was equivalent in WT and tnfr1-/- grafts, the overall abundance of cells was substantially greater in WT grafts. WT grafts demonstrated greater MCP-1, vascular cell adhesion molecule-1, and intercellular adhesion molecule-1 expression at both early and late time points, and proliferating cell nuclear antigen expression at early time points. Aortic atherosclerosis was also reduced in 14-month-old apoe(-/-)/tnfr1(-/-) mice, as compared with cognate apoe-/- mice. In coculture with activated macrophages, smooth muscle cells expressing the TNFR1 demonstrated enhanced migration and reduced scavenger receptor activity.
Conclusions: TNFR1 signaling, just in arterial wall cells, contributes to the pathogenesis of atherosclerosis by enhancing arterial wall chemokine and adhesion molecule expression, as well as by augmenting medial smooth muscle cell proliferation and migration.
Figures


Similar articles
-
Vein graft neointimal hyperplasia is exacerbated by tumor necrosis factor receptor-1 signaling in graft-intrinsic cells.Arterioscler Thromb Vasc Biol. 2004 Dec;24(12):2277-83. doi: 10.1161/01.ATV.0000147766.68987.0d. Epub 2004 Oct 14. Arterioscler Thromb Vasc Biol. 2004. PMID: 15486311
-
Aging-related atherosclerosis is exacerbated by arterial expression of tumor necrosis factor receptor-1: evidence from mouse models and human association studies.Hum Mol Genet. 2010 Jul 15;19(14):2754-66. doi: 10.1093/hmg/ddq172. Epub 2010 Apr 26. Hum Mol Genet. 2010. PMID: 20421368 Free PMC article.
-
Tumor necrosis factor receptor-2 signaling attenuates vein graft neointima formation by promoting endothelial recovery.Arterioscler Thromb Vasc Biol. 2008 Feb;28(2):284-9. doi: 10.1161/ATVBAHA.107.151613. Epub 2007 Nov 15. Arterioscler Thromb Vasc Biol. 2008. PMID: 18006858
-
Atheroprotective role of C5ar2 deficiency in apolipoprotein E-deficient mice.Thromb Haemost. 2015 Oct;114(4):848-58. doi: 10.1160/TH14-12-1075. Epub 2015 Jun 18. Thromb Haemost. 2015. PMID: 26084965
-
Local Vascular Gene Therapy With Apolipoprotein A-I to Promote Regression of Atherosclerosis.Arterioscler Thromb Vasc Biol. 2017 Feb;37(2):316-327. doi: 10.1161/ATVBAHA.116.308258. Epub 2016 Dec 8. Arterioscler Thromb Vasc Biol. 2017. PMID: 27932352 Free PMC article.
Cited by
-
Multiplex Protein Biomarker Profiling in Patients with Familial Hypercholesterolemia.Genes (Basel). 2021 Oct 12;12(10):1599. doi: 10.3390/genes12101599. Genes (Basel). 2021. PMID: 34680994 Free PMC article.
-
Berry-Derived Polyphenols in Cardiovascular Pathologies: Mechanisms of Disease and the Role of Diet and Sex.Nutrients. 2021 Jan 27;13(2):387. doi: 10.3390/nu13020387. Nutrients. 2021. PMID: 33513742 Free PMC article. Review.
-
Improving the prediction of intracranial aneurysm rupture risk-calcification in the spotlight.Eur Radiol. 2024 Nov;34(11):7514-7516. doi: 10.1007/s00330-024-10990-3. Epub 2024 Jul 29. Eur Radiol. 2024. PMID: 39075301 No abstract available.
-
Adipokines, adiposity, and atherosclerosis.Cell Mol Life Sci. 2022 May 3;79(5):272. doi: 10.1007/s00018-022-04286-2. Cell Mol Life Sci. 2022. PMID: 35503385 Free PMC article. Review.
-
MicroRNA-29a-3p Reduces TNFα-Induced Endothelial Dysfunction by Targeting Tumor Necrosis Factor Receptor 1.Mol Ther Nucleic Acids. 2019 Dec 6;18:903-915. doi: 10.1016/j.omtn.2019.10.014. Epub 2019 Oct 22. Mol Ther Nucleic Acids. 2019. PMID: 31760375 Free PMC article.
References
-
- Branen L, Hovgaard L, Nitulescu M, Bengtsson E, Nilsson J, Jovinge S. Inhibition of tumor necrosis factor-α reduces atherosclerosis in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2004;24:2137–2142. - PubMed
-
- Ridker PM, Rifai N, Pfeffer M, Sacks F, Lepage S, Braunwald E. Elevation of tumor necrosis factor-alpha and increased risk of recurrent coronary events after myocardial infarction. Circulation. 2000;101:2149–2153. - PubMed
-
- Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–454. - PubMed
-
- Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–979. - PubMed
-
- Stannard AK, Riddell DR, Bradley NJ, Hassall DG, Graham A, Owen JS. Apolipoprotein E and regulation of cytokine-induced cell adhesion molecule expression in endothelial cells. Atherosclerosis. 1998;139:57–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AG25462/AG/NIA NIH HHS/United States
- R01 HL072842-03/HL/NHLBI NIH HHS/United States
- R01 HL073005-01A3/HL/NHLBI NIH HHS/United States
- R01 HL073005-02/HL/NHLBI NIH HHS/United States
- R01 HL073005-03/HL/NHLBI NIH HHS/United States
- R01 HL072842-01A2/HL/NHLBI NIH HHS/United States
- HL73005/HL/NHLBI NIH HHS/United States
- R21 AG025462/AG/NIA NIH HHS/United States
- R01 HL073005/HL/NHLBI NIH HHS/United States
- R21 AG025462-01A1/AG/NIA NIH HHS/United States
- R01 HL072842/HL/NHLBI NIH HHS/United States
- HL72842/HL/NHLBI NIH HHS/United States
- R01 HL072842-02/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

