Functional interaction between phosducin-like protein 2 and cytosolic chaperonin is essential for cytoskeletal protein function and cell cycle progression
- PMID: 17429077
- PMCID: PMC1877119
- DOI: 10.1091/mbc.e07-01-0069
Functional interaction between phosducin-like protein 2 and cytosolic chaperonin is essential for cytoskeletal protein function and cell cycle progression
Abstract
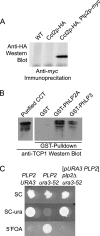
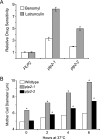
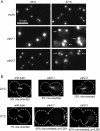
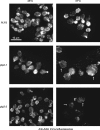
The Chaperonin Containing Tcp1 (CCT) maintains cellular protein folding homeostasis in the eukaryotic cytosol by assisting the biogenesis of many proteins, including actins, tubulins, and regulators of the cell cycle. Here, we demonstrate that the essential and conserved eukaryotic phosducin-like protein 2 (PhLP2/PLP2) physically interacts with CCT and modulates its folding activity. Consistent with this functional interaction, temperature-sensitive alleles of Saccharomyces cerevisiae PLP2 exhibit cytoskeletal and cell cycle defects. We uncovered several high-copy suppressors of the plp2 alleles, all of which are associated with G1/S cell cycle progression but which do not appreciably affect cytoskeletal protein function or fully rescue the growth defects. Our data support a model in which Plp2p modulates the biogenesis of several CCT substrates relating to cell cycle and cytoskeletal function, which together contribute to the essential function of PLP2.
Figures


Similar articles
-
Yeast phosducin-like protein 2 acts as a stimulatory co-factor for the folding of actin by the chaperonin CCT via a ternary complex.J Mol Biol. 2009 Aug 7;391(1):192-206. doi: 10.1016/j.jmb.2009.06.003. Epub 2009 Jun 6. J Mol Biol. 2009. PMID: 19501098
-
ATP-induced allostery in the eukaryotic chaperonin CCT is abolished by the mutation G345D in CCT4 that renders yeast temperature-sensitive for growth.J Mol Biol. 2008 Mar 21;377(2):469-77. doi: 10.1016/j.jmb.2008.01.011. Epub 2008 Jan 15. J Mol Biol. 2008. PMID: 18272176
-
The essential yeast Tcp1 protein affects actin and microtubules.Mol Biol Cell. 1994 Oct;5(10):1065-80. doi: 10.1091/mbc.5.10.1065. Mol Biol Cell. 1994. PMID: 7865875 Free PMC article.
-
Function of phosducin-like proteins in G protein signaling and chaperone-assisted protein folding.Cell Signal. 2007 Dec;19(12):2417-27. doi: 10.1016/j.cellsig.2007.06.013. Epub 2007 Jun 28. Cell Signal. 2007. PMID: 17658730 Free PMC article. Review.
-
Quality control of cytoskeletal proteins and human disease.Trends Biochem Sci. 2010 May;35(5):288-97. doi: 10.1016/j.tibs.2009.12.007. Epub 2010 Jan 28. Trends Biochem Sci. 2010. PMID: 20116259 Review.
Cited by
-
Misato Controls Mitotic Microtubule Generation by Stabilizing the TCP-1 Tubulin Chaperone Complex [corrected].Curr Biol. 2015 Jun 29;25(13):1777-83. doi: 10.1016/j.cub.2015.05.033. Epub 2015 Jun 18. Curr Biol. 2015. PMID: 26096973 Free PMC article.
-
The structural basis of eukaryotic chaperonin TRiC/CCT: Action and folding.Mol Cells. 2024 Mar;47(3):100012. doi: 10.1016/j.mocell.2024.100012. Epub 2024 Jan 26. Mol Cells. 2024. PMID: 38280673 Free PMC article. Review.
-
Gene expression profiling of substantia nigra dopamine neurons: further insights into Parkinson's disease pathology.Brain. 2009 Jul;132(Pt 7):1795-809. doi: 10.1093/brain/awn323. Epub 2008 Dec 3. Brain. 2009. PMID: 19052140 Free PMC article.
-
The evolutionarily conserved PhLP3 is essential for sperm development in Drosophila melanogaster.PLoS One. 2024 Oct 31;19(10):e0306676. doi: 10.1371/journal.pone.0306676. eCollection 2024. PLoS One. 2024. PMID: 39480878 Free PMC article.
-
Functional Subunits of Eukaryotic Chaperonin CCT/TRiC in Protein Folding.J Amino Acids. 2011;2011:843206. doi: 10.4061/2011/843206. Epub 2011 Jul 2. J Amino Acids. 2011. PMID: 22312474 Free PMC article.
References
-
- Adams A., Gottschling D. E., Kaiser C. A., Stearns T. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1997. Methods in Yeast Genetics: A Cold Spring Harbor Laboratory Course Manual.
-
- Amberg D. C., Burke D. J., Strathern J. N. 2005 ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2005. Methods in Yeast Genetics.
-
- Camasses A., Bogdanova A., Shevchenko A., Zachariae W. The CCT chaperonin promotes activation of the anaphase-promoting complex through the generation of functional Cdc20. Mol. Cell. 2003;12:87–100. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

