Cardiac myocyte cell cycle control in development, disease, and regeneration
- PMID: 17429040
- PMCID: PMC2708177
- DOI: 10.1152/physrev.00032.2006
Cardiac myocyte cell cycle control in development, disease, and regeneration
Abstract
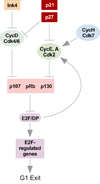
Cardiac myocytes rapidly proliferate during fetal life but exit the cell cycle soon after birth in mammals. Although the extent to which adult cardiac myocytes are capable of cell cycle reentry is controversial and species-specific differences may exist, it appears that for the vast majority of adult cardiac myocytes the predominant form of growth postnatally is an increase in cell size (hypertrophy) not number. Unfortunately, this limits the ability of the heart to restore function after any significant injury. Interest in novel regenerative therapies has led to the accumulation of much information on the mechanisms that regulate the rapid proliferation of cardiac myocytes in utero, their cell cycle exit in the perinatal period, and the permanent arrest (terminal differentiation) in adult myocytes. The recent identification of cardiac progenitor cells capable of giving rise to cardiac myocyte-like cells has challenged the dogma that the heart is a terminally differentiated organ and opened new prospects for cardiac regeneration. In this review, we summarize the current understanding of cardiomyocyte cell cycle control in normal development and disease. In addition, we also discuss the potential usefulness of cardiomyocyte self-renewal as well as feasibility of therapeutic manipulation of the cardiac myocyte cell cycle for cardiac regeneration.
Figures
Similar articles
-
Multimodal Regulation of Cardiac Myocyte Proliferation.Circ Res. 2017 Jul 21;121(3):293-309. doi: 10.1161/CIRCRESAHA.117.308428. Circ Res. 2017. PMID: 28729454 Review.
-
The cardiomyocyte cell cycle in hypertrophy, tissue homeostasis, and regeneration.Rev Physiol Biochem Pharmacol. 2013;165:67-96. doi: 10.1007/112_2013_12. Rev Physiol Biochem Pharmacol. 2013. PMID: 23605180 Review.
-
Cardiomyocyte Proliferation as a Source of New Myocyte Development in the Adult Heart.Int J Mol Sci. 2021 Jul 21;22(15):7764. doi: 10.3390/ijms22157764. Int J Mol Sci. 2021. PMID: 34360531 Free PMC article. Review.
-
Cardiac Regeneration and Stem Cells.Physiol Rev. 2015 Oct;95(4):1189-204. doi: 10.1152/physrev.00021.2014. Physiol Rev. 2015. PMID: 26269526 Free PMC article. Review.
-
Regulation of cardiomyocyte proliferation during development and regeneration.Dev Growth Differ. 2014 Jun;56(5):402-9. doi: 10.1111/dgd.12134. Epub 2014 Apr 16. Dev Growth Differ. 2014. PMID: 24738847 Review.
Cited by
-
Linking metabolic dysfunction with cardiovascular diseases: Brn-3b/POU4F2 transcription factor in cardiometabolic tissues in health and disease.Cell Death Dis. 2021 Mar 12;12(3):267. doi: 10.1038/s41419-021-03551-9. Cell Death Dis. 2021. PMID: 33712567 Free PMC article. Review.
-
Loss of Rbl2 (Retinoblastoma-Like 2) Exacerbates Myocardial Ischemia/Reperfusion Injury.J Am Heart Assoc. 2022 Oct 4;11(19):e024764. doi: 10.1161/JAHA.121.024764. Epub 2022 Sep 21. J Am Heart Assoc. 2022. PMID: 36129061 Free PMC article.
-
The periconceptional environment and cardiovascular disease: does in vitro embryo culture and transfer influence cardiovascular development and health?Nutrients. 2015 Feb 18;7(3):1378-425. doi: 10.3390/nu7031378. Nutrients. 2015. PMID: 25699984 Free PMC article. Review.
-
collectNET: a web server for integrated inference of cell-cell communication network.Database (Oxford). 2024 Sep 16;2024:baae098. doi: 10.1093/database/baae098. Database (Oxford). 2024. PMID: 39283594 Free PMC article.
-
Cardiomyocyte proliferation in zebrafish and mammals: lessons for human disease.Cell Mol Life Sci. 2017 Apr;74(8):1367-1378. doi: 10.1007/s00018-016-2404-x. Epub 2016 Nov 3. Cell Mol Life Sci. 2017. PMID: 27812722 Free PMC article. Review.
References
-
- Identification and characterization of the tuberous sclerosis gene on chromosome 16. The European Chromosome 16 Tuberous Sclerosis Consortium. Cell. 1993;75:1305–1315. - PubMed
-
- Abdullah I, Lepore JJ, Epstein JA, Parmacek MS, Gruber PJ. MRL mice fail to heal the heart in response to ischemia-reperfusion injury. Wound.Repair Regen. 2005;13:205–208. - PubMed
-
- Ahuja P, Perriard E, Perriard JC, Ehler E. Sequential myofibrillar breakdown accompanies mitotic division of mammalian cardiomyocytes. J.Cell Sci. 2004;117:3295–3306. - PubMed
-
- Ahuja P, Perriard E, Trimble W, Perriard JC, Ehler E. Probing the role of septins in cardiomyocytes. Exp.Cell Res. 2006;312:1598–1609. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

