A role for cell sex in stem cell-mediated skeletal muscle regeneration: female cells have higher muscle regeneration efficiency
- PMID: 17420291
- PMCID: PMC2064113
- DOI: 10.1083/jcb.200612094
A role for cell sex in stem cell-mediated skeletal muscle regeneration: female cells have higher muscle regeneration efficiency
Abstract
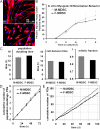
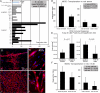
We have shown that muscle-derived stem cells (MDSCs) transplanted into dystrophic (mdx) mice efficiently regenerate skeletal muscle. However, MDSC populations exhibit heterogeneity in marker profiles and variability in regeneration abilities. We show here that cell sex is a variable that considerably influences MDSCs' regeneration abilities. We found that the female MDSCs (F-MDSCs) regenerated skeletal muscle more efficiently. Despite using additional isolation techniques and cell cloning, we could not obtain a male subfraction with a regeneration capacity similar to that of their female counterparts. Rather than being directly hormonal or caused by host immune response, this difference in MDSCs' regeneration potential may arise from innate sex-related differences in the cells' stress responses. In comparison with F-MDSCs, male MDSCs have increased differentiation after exposure to oxidative stress induced by hydrogen peroxide, which may lead to in vivo donor cell depletion, and a proliferative advantage for F-MDSCs that eventually increases muscle regeneration. These findings should persuade researchers to report cell sex, which is a largely unexplored variable, and consider the implications of relying on cells of one sex.
Figures

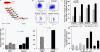


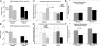
Similar articles
-
The influence of sex on the chondrogenic potential of muscle-derived stem cells: implications for cartilage regeneration and repair.Arthritis Rheum. 2008 Dec;58(12):3809-19. doi: 10.1002/art.24125. Arthritis Rheum. 2008. PMID: 19035511
-
Nerve growth factor improves the muscle regeneration capacity of muscle stem cells in dystrophic muscle.Hum Gene Ther. 2006 Feb;17(2):180-92. doi: 10.1089/hum.2006.17.180. Hum Gene Ther. 2006. PMID: 16454652
-
Beneficial effect of mechanical stimulation on the regenerative potential of muscle-derived stem cells is lost by inhibiting vascular endothelial growth factor.Arterioscler Thromb Vasc Biol. 2013 Aug;33(8):2004-12. doi: 10.1161/ATVBAHA.112.301166. Epub 2013 May 30. Arterioscler Thromb Vasc Biol. 2013. PMID: 23723372 Free PMC article.
-
Muscle-derived stem cells: isolation, characterization, differentiation, and application in cell and gene therapy.Cell Tissue Res. 2010 Jun;340(3):549-67. doi: 10.1007/s00441-010-0978-4. Epub 2010 May 22. Cell Tissue Res. 2010. PMID: 20495827 Review.
-
Muscle-derived stem cells for musculoskeletal tissue regeneration and repair.Transpl Immunol. 2004 Apr;12(3-4):311-9. doi: 10.1016/j.trim.2003.12.009. Transpl Immunol. 2004. PMID: 15157924 Review.
Cited by
-
Increased fat deposition in injured skeletal muscle is regulated by sex-specific hormones.Am J Physiol Regul Integr Comp Physiol. 2012 Feb 1;302(3):R331-9. doi: 10.1152/ajpregu.00427.2011. Epub 2011 Nov 23. Am J Physiol Regul Integr Comp Physiol. 2012. PMID: 22116509 Free PMC article.
-
Total testosterone is not associated with lean mass or handgrip strength in pre-menopausal females.Sci Rep. 2021 May 13;11(1):10226. doi: 10.1038/s41598-021-89232-1. Sci Rep. 2021. PMID: 33986323 Free PMC article.
-
Solving for (se)x.Lab Anim (NY). 2015 Oct;44(10):371-2. doi: 10.1038/laban.876. Lab Anim (NY). 2015. PMID: 26398603 No abstract available.
-
Evaluation of phenotypic, functional and molecular characteristics of porcine mesenchymal stromal/stem cells depending on donor age, gender and tissue source.J Vet Med Sci. 2016 Jul 1;78(6):987-95. doi: 10.1292/jvms.15-0596. Epub 2016 Feb 26. J Vet Med Sci. 2016. PMID: 26922917 Free PMC article.
-
Intrinsic sex-specific differences in microvascular endothelial cell phosphodiesterases.Am J Physiol Heart Circ Physiol. 2010 Apr;298(4):H1146-54. doi: 10.1152/ajpheart.00252.2009. Epub 2010 Feb 5. Am J Physiol Heart Circ Physiol. 2010. PMID: 20139324 Free PMC article.
References
-
- Aristotle. 350 BC. Historia Animalium: Books VII–X. 1991. edition. D.M. Balme, editor. Harvard University Press, Cambridge, MA. 435–437.
-
- Avery, B., C.B. Jorgensen, V. Madison, and T. Greve. 1992. Morphological development and sex of bovine in vitro-fertilized embryos. Mol. Reprod. Dev. 32:265–270. - PubMed
-
- Aviv, A. 2002. Telomeres, sex, reactive oxygen species, and human cardiovascular aging. J. Mol. Med. 80:689–695. - PubMed
-
- Aviv, A., J. Shay, K. Christensen, and W. Wright. 2005. The longevity gender gap: are telomeres the explanation? Sci. Aging Knowledge Environ. 2005:pe16. - PubMed
-
- Beauchamp, J.R., J.E. Morgan, C.N. Pagel, and T.A. Partridge. 1994. Quantitative studies of efficacy of myoblast transplantation. Muscle Nerve. 1:S261.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

