Adenoviral gene transfer of bioactive TGFbeta1 to the rodent eye as a novel model for anterior subcapsular cataract
- PMID: 17417606
- PMCID: PMC2647562
Adenoviral gene transfer of bioactive TGFbeta1 to the rodent eye as a novel model for anterior subcapsular cataract
Abstract
Purpose: To produce a gene-transfer model of rodent anterior subcapsular cataracts (ASC) using a replication-deficient, adenoviral vector containing active TGFbeta1. Establishment of this model will be important for further investigations of TGFbeta-induced signaling cascades in ASC.
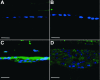
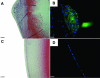
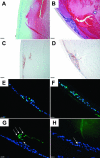
Methods: Adenovirus containing the transgene for active TGFbeta1 (AdTGFbeta1), beta-galactosidase (AdLacZ), green fluorescent protein (AdGFP) or no transgene (AdDL) was injected into the anterior chamber of C57Bl/6, Smad3 WT and Smad3 KO mice. Four and 21 days after injection, animals were enucleated and eyes were processed and examined by routine histology. Immunolocalization of markers indicative of epithelial to mesenchymal transition (EMT), fibrosis, proliferation and apoptosis was also carried out.
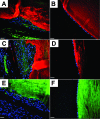
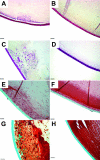
Results: By day 4, treatment with AdLacZ demonstrated transgene expression in multiple structures of the anterior chamber including the lens epithelium. In contrast to AdDL, treatment with AdTGFbeta1 produced alphaSMA-positive subcapsular plaques in all three groups of mice, which shared features reminiscent of human ASC. At day 21, plaques remained alphaSMA-positive and extensive extracellular matrix deposition was observed. The AdTGFbeta1 model was further employed in Smad3 deficient mice and this resulted in the development of small ASC.
Conclusions: Gene transfer of active TGFbeta1 using an adenoviral vector produced cataractous plaques four days postinjection, which were found to develop independent of functional Smad3.
Figures



Similar articles
-
Lens-specific expression of TGF-beta induces anterior subcapsular cataract formation in the absence of Smad3.Invest Ophthalmol Vis Sci. 2006 Aug;47(8):3450-60. doi: 10.1167/iovs.05-1208. Invest Ophthalmol Vis Sci. 2006. PMID: 16877415 Free PMC article.
-
Aberrant lens fiber differentiation in anterior subcapsular cataract formation: a process dependent on reduced levels of Pax6.Invest Ophthalmol Vis Sci. 2004 Jun;45(6):1946-53. doi: 10.1167/iovs.03-1206. Invest Ophthalmol Vis Sci. 2004. PMID: 15161862
-
A new model of anterior subcapsular cataract: involvement of TGFbeta/Smad signaling.Mol Vis. 2006 Jun 14;12:681-91. Mol Vis. 2006. PMID: 16807527
-
Transforming growth factor-beta-induced epithelial-mesenchymal transition in the lens: a model for cataract formation.Cells Tissues Organs. 2005;179(1-2):43-55. doi: 10.1159/000084508. Cells Tissues Organs. 2005. PMID: 15942192 Review.
-
Fibrosis in the lens. Sprouty regulation of TGFβ-signaling prevents lens EMT leading to cataract.Exp Eye Res. 2016 Jan;142:92-101. doi: 10.1016/j.exer.2015.02.004. Epub 2015 May 21. Exp Eye Res. 2016. PMID: 26003864 Free PMC article. Review.
Cited by
-
Altered expression of transforming growth factor beta 1 and matrix metalloproteinase-9 results in elevated intraocular pressure in mice.Mol Vis. 2013;19:684-95. Epub 2013 Mar 21. Mol Vis. 2013. PMID: 23559862 Free PMC article.
-
MYPT1/PP1-Mediated EZH2 Dephosphorylation at S21 Promotes Epithelial-Mesenchymal Transition in Fibrosis through Control of Multiple Families of Genes.Adv Sci (Weinh). 2022 May;9(14):e2105539. doi: 10.1002/advs.202105539. Epub 2022 Mar 16. Adv Sci (Weinh). 2022. PMID: 35293697 Free PMC article.
-
The lens capsule.Exp Eye Res. 2009 Feb;88(2):151-64. doi: 10.1016/j.exer.2008.08.002. Epub 2008 Aug 16. Exp Eye Res. 2009. PMID: 18773892 Free PMC article. Review.
-
The local wound environment is a key determinant of the outcome of TGFβ signaling on the fibrotic response of CD44+ leader cells in an ex vivo post-cataract-surgery model.Exp Eye Res. 2021 Dec;213:108829. doi: 10.1016/j.exer.2021.108829. Epub 2021 Nov 10. Exp Eye Res. 2021. PMID: 34774488 Free PMC article.
-
Let-7a-5p represses proliferation, migration, invasion and epithelial-mesenchymal transition by targeting Smad2 in TGF-b2-induced human lens epithelial cells.J Biosci. 2020;45:59. J Biosci. 2020. PMID: 32345785
References
-
- Derynck R. TGF-beta-receptor-mediated signaling. Trends Biochem Sci. 1994;19:548–53. - PubMed
-
- Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J. 2004;18:816–27. - PubMed
-
- Saika S. TGFbeta pathobiology in the eye. Lab Invest. 2006;86:106–15. - PubMed
-
- Saika S, Yamanaka O, Ikeda K, Kim-Mitsuyama S, Flanders KC, Yoo J, Roberts AB, Nishikawa-Ishida I, Ohnishi Y, Muragaki Y, Ooshima A. Inhibition of p38MAP kinase suppresses fibrotic reaction of retinal pigment epithelial cells. Lab Invest. 2005;85:838–50. - PubMed
-
- Priglinger SG, Alge CS, Kreutzer TC, Neubauer AS, Haritoglou C, Kampik A, Welge-Luessen U. Keratinocyte transglutaminase in proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci. 2006;47:4990–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
