The cytomegalovirus-specific CD4+ T-cell response expands with age and markedly alters the CD4+ T-cell repertoire
- PMID: 17409149
- PMCID: PMC1933343
- DOI: 10.1128/JVI.01262-06
The cytomegalovirus-specific CD4+ T-cell response expands with age and markedly alters the CD4+ T-cell repertoire
Abstract
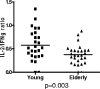
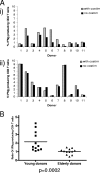
Immune function in the elderly is associated with a number of phenotypic and functional abnormalities, and this phenomenon of immune senescence is associated with increased susceptibility to infection. The immune response to pathogens frequently declines with age, but the CD8(+) T-cell response to cytomegalovirus (CMV) is unusual, as it demonstrates a significant expansion over time. Here we have documented the CD4(+) T-cell immune response to CMV in healthy donors of different ages. The magnitude of the CMV-specific CD4(+) T-cell immune response increases from a mean of 2.2% of the CD4(+) T-cell pool in donors below 50 years of age to 4.7% in donors aged over 65 years. In addition, CMV-specific CD4(+) T cells in elderly donors demonstrate decreased production of interleukin-2 and less dependence on costimulation. CMV seropositivity is associated with marked changes in the phenotype of the overall CD4(+) T-cell repertoire in healthy aged donors, including an increase in CD57(+) expression and a decrease in CD28 and CD27 expression, a phenotypic profile characteristic of immune senescence. This memory inflation of CMV-specific CD4(+) T cells contributes to evidence that CMV infection may be damaging to immune function in elderly individuals.
Figures


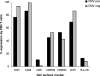
Similar articles
-
Immune resilience in HIV-infected individuals seronegative for cytomegalovirus.AIDS. 2014 Sep 10;28(14):2045-9. doi: 10.1097/QAD.0000000000000405. AIDS. 2014. PMID: 25265072
-
Protective immunity to cytomegalovirus (CMV) retinitis in AIDS is associated with CMV-specific T cells that express interferon- gamma and interleukin-2 and have a CD8+ cell early maturational phenotype.J Infect Dis. 2006 Dec 1;194(11):1537-46. doi: 10.1086/508997. Epub 2006 Oct 26. J Infect Dis. 2006. PMID: 17083038
-
Healthy aging and latent infection with CMV lead to distinct changes in CD8+ and CD4+ T-cell subsets in the elderly.Hum Immunol. 2007 Feb;68(2):86-90. doi: 10.1016/j.humimm.2006.10.019. Epub 2006 Dec 5. Hum Immunol. 2007. PMID: 17321897
-
Analyzing T-cell responses to cytomegalovirus by cytokine flow cytometry.Hum Immunol. 2004 May;65(5):493-9. doi: 10.1016/j.humimm.2004.02.004. Hum Immunol. 2004. PMID: 15172449 Review.
-
The Immune Response Against Human Cytomegalovirus Links Cellular to Systemic Senescence.Cells. 2020 Mar 20;9(3):766. doi: 10.3390/cells9030766. Cells. 2020. PMID: 32245117 Free PMC article. Review.
Cited by
-
Depressive symptoms post hip fracture in older adults are associated with phenotypic and functional alterations in T cells.Immun Ageing. 2014 Dec 16;11(1):25. doi: 10.1186/s12979-014-0025-5. eCollection 2014. Immun Ageing. 2014. PMID: 25628751 Free PMC article.
-
Cytomegalovirus Infection Leads to Development of High Frequencies of Cytotoxic Virus-Specific CD4+ T Cells Targeted to Vascular Endothelium.PLoS Pathog. 2016 Sep 8;12(9):e1005832. doi: 10.1371/journal.ppat.1005832. eCollection 2016 Sep. PLoS Pathog. 2016. PMID: 27606804 Free PMC article.
-
Chronic lymphocytic leukemia patients have a preserved cytomegalovirus-specific antibody response despite progressive hypogammaglobulinemia.PLoS One. 2013 Oct 23;8(10):e78925. doi: 10.1371/journal.pone.0078925. eCollection 2013. PLoS One. 2013. PMID: 24194956 Free PMC article.
-
Human Macrophages Escape Inhibition of Major Histocompatibility Complex-Dependent Antigen Presentation by Cytomegalovirus and Drive Proliferation and Activation of Memory CD4+ and CD8+ T Cells.Front Immunol. 2018 May 25;9:1129. doi: 10.3389/fimmu.2018.01129. eCollection 2018. Front Immunol. 2018. PMID: 29887865 Free PMC article.
-
Successful and Maladaptive T Cell Aging.Immunity. 2017 Mar 21;46(3):364-378. doi: 10.1016/j.immuni.2017.03.010. Immunity. 2017. PMID: 28329703 Free PMC article. Review.
References
-
- Almanzar G., S. Schwaiger, B. Jenewein, M. Keller, D. Herndler-Brandstetter, R. Wurzner, D. Schonitzer, and B. Grubeck-Loebenstein. 2005. Long-term cytomegalovirus infection leads to significant changes in the composition of the CD8+ T-cell repertoire, which may be the basis for an imbalance in the cytokine production profile in elderly persons. J. Virol. 79:3675-3683. - PMC - PubMed
-
- Andersson, E., M. Ohlin, C. A. Borrebaeck, and R. Carlsson. 1995. CD4+ CD57+ T cells derived from peripheral blood do not support immunoglobulin production by B cells. Cell. Immunol. 163:245-253. - PubMed
-
- Asanuma, H., M. Sharp, H. T. Maecker, V. C. Maino, and A. M. Arvin. 2000. Frequencies of memory T cells specific for varicella-zoster virus, herpes simplex virus, and cytomegalovirus by intracellular detection of cytokine expression. J. Infect. Dis. 181:859-866. - PubMed
-
- Baars, P. A., M. M. Maurice, M. Rep, B. Hooibrink, and R. A. van Lier. 1995. Heterogeneity of the circulating human CD4+ T cell population. Further evidence that the CD4+ CD45RA− CD27− T cell subset contains specialized primed T cells. J. Immunol. 154:17-25. - PubMed
-
- Bernstein, E., D. Kaye, E. Abrutyn, P. Gross, M. Dorfman, and D. M. Murasko. 1999. Immune response to influenza vaccination in a large healthy elderly population. Vaccine 17:82-94. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

