Rubisco large-subunit translation is autoregulated in response to its assembly state in tobacco chloroplasts
- PMID: 17404229
- PMCID: PMC1851044
- DOI: 10.1073/pnas.0610586104
Rubisco large-subunit translation is autoregulated in response to its assembly state in tobacco chloroplasts
Abstract
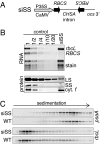
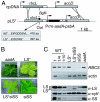
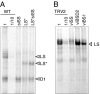
Plants rely on ribulose bisphosphate carboxylase/oxygenase (Rubisco) for carbon fixation. Higher plant Rubisco possesses an L(8)S(8) structure, with the large subunit (LS) encoded in the chloroplast by rbcL and the small subunit encoded by the nuclear RBCS gene family. Because its components accumulate stoichiometrically but are encoded in two genetic compartments, rbcL and RBCS expression must be tightly coordinated. Although this coordination has been observed, the underlying mechanisms have not been defined. Here, we use tobacco to understand how LS translation is related to its assembly status. To do so, two transgenic lines deficient in LS biogenesis were created: a chloroplast transformant expressing a truncated and unstable LS polypeptide, and a line where a homolog of the maize Rubisco-specific chaperone, BSD2, was repressed by RNAi. We found that in both lines, LS translation is no longer regulated by the availability of small subunit (SS), indicating that LS translation is not activated by the presence of its assembly partner but, rather, undergoes an autoregulation of translation. Pulse labeling experiments indicate that LS is synthesized but not accumulated in the transgenic lines, suggesting that accumulation of a repressor motif is required for LS assembly-dependent translational regulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Ribulose 1,5-bisphosphate carboxylase/oxygenase large subunit translation is regulated in a small subunit-independent manner in the expanded leaves of tobacco.Plant Cell Physiol. 2008 Feb;49(2):214-25. doi: 10.1093/pcp/pcm179. Epub 2008 Jan 4. Plant Cell Physiol. 2008. PMID: 18178584
-
A mechanism for intergenomic integration: abundance of ribulose bisphosphate carboxylase small-subunit protein influences the translation of the large-subunit mRNA.Proc Natl Acad Sci U S A. 1996 Apr 30;93(9):3881-5. doi: 10.1073/pnas.93.9.3881. Proc Natl Acad Sci U S A. 1996. PMID: 8632983 Free PMC article.
-
Ectopic expression of Rubisco subunits in maize mesophyll cells does not overcome barriers to cell type-specific accumulation.Plant Physiol. 2012 Sep;160(1):419-32. doi: 10.1104/pp.112.195677. Epub 2012 Jun 28. Plant Physiol. 2012. PMID: 22744982 Free PMC article.
-
Regulation of Rubisco gene expression in C4 plants.Curr Opin Plant Biol. 2016 Jun;31:23-8. doi: 10.1016/j.pbi.2016.03.004. Epub 2016 Mar 26. Curr Opin Plant Biol. 2016. PMID: 27026038 Review.
-
Manipulating ribulose bisphosphate carboxylase/oxygenase in the chloroplasts of higher plants.Arch Biochem Biophys. 2003 Jun 15;414(2):159-69. doi: 10.1016/s0003-9861(03)00100-0. Arch Biochem Biophys. 2003. PMID: 12781767 Review.
Cited by
-
Modifying Plant Photosynthesis and Growth via Simultaneous Chloroplast Transformation of Rubisco Large and Small Subunits.Plant Cell. 2020 Sep;32(9):2898-2916. doi: 10.1105/tpc.20.00288. Epub 2020 Jul 9. Plant Cell. 2020. PMID: 32647068 Free PMC article.
-
Absolute Quantification of Major Photosynthetic Protein Complexes in Chlamydomonas reinhardtii Using Quantification Concatamers (QconCATs).Front Plant Sci. 2018 Aug 30;9:1265. doi: 10.3389/fpls.2018.01265. eCollection 2018. Front Plant Sci. 2018. PMID: 30214453 Free PMC article.
-
Advancing our understanding and capacity to engineer nature's CO2-sequestering enzyme, Rubisco.Plant Physiol. 2011 Jan;155(1):27-35. doi: 10.1104/pp.110.164814. Epub 2010 Oct 25. Plant Physiol. 2011. PMID: 20974895 Free PMC article. No abstract available.
-
Exploring mechanisms linked to differentiation and function of dimorphic chloroplasts in the single cell C4 species Bienertia sinuspersici.BMC Plant Biol. 2014 Jan 21;14:34. doi: 10.1186/1471-2229-14-34. BMC Plant Biol. 2014. PMID: 24443986 Free PMC article.
-
PPR proteins of green algae.RNA Biol. 2013;10(9):1526-42. doi: 10.4161/rna.26127. Epub 2013 Aug 28. RNA Biol. 2013. PMID: 24021981 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

