Phosphorylation and regulation of a G protein-coupled receptor by protein kinase CK2
- PMID: 17403928
- PMCID: PMC2064117
- DOI: 10.1083/jcb.200610018
Phosphorylation and regulation of a G protein-coupled receptor by protein kinase CK2
Abstract
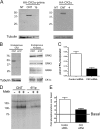
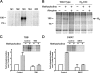
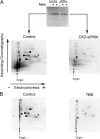
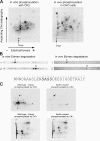
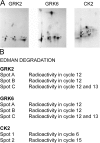
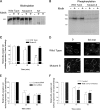
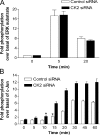
We demonstrate a role for protein kinase casein kinase 2 (CK2) in the phosphorylation and regulation of the M3-muscarinic receptor in transfected cells and cerebellar granule neurons. On agonist occupation, specific subsets of receptor phosphoacceptor sites (which include the SASSDEED motif in the third intracellular loop) are phosphorylated by CK2. Receptor phosphorylation mediated by CK2 specifically regulates receptor coupling to the Jun-kinase pathway. Importantly, other phosphorylation-dependent receptor processes are regulated by kinases distinct from CK2. We conclude that G protein-coupled receptors (GPCRs) can be phosphorylated in an agonist-dependent fashion by protein kinases from a diverse range of kinase families, not just the GPCR kinases, and that receptor phosphorylation by a defined kinase determines a specific signalling outcome. Furthermore, we demonstrate that the M3-muscarinic receptor can be differentially phosphorylated in different cell types, indicating that phosphorylation is a flexible regulatory process where the sites that are phosphorylated, and hence the signalling outcome, are dependent on the cell type in which the receptor is expressed.
Figures


Similar articles
-
Stimulus-dependent phosphorylation of G-protein-coupled receptors by casein kinase 1alpha.J Biol Chem. 1997 Aug 15;272(33):20844-9. doi: 10.1074/jbc.272.33.20844. J Biol Chem. 1997. PMID: 9252410
-
Specificity of g protein-coupled receptor kinase 6-mediated phosphorylation and regulation of single-cell m3 muscarinic acetylcholine receptor signaling.Mol Pharmacol. 2003 Nov;64(5):1059-68. doi: 10.1124/mol.64.5.1059. Mol Pharmacol. 2003. PMID: 14573754
-
CK2 acts as a potent negative regulator of receptor-mediated insulin release in vitro and in vivo.Proc Natl Acad Sci U S A. 2015 Dec 8;112(49):E6818-24. doi: 10.1073/pnas.1519430112. Epub 2015 Nov 23. Proc Natl Acad Sci U S A. 2015. PMID: 26598688 Free PMC article.
-
Protein kinase CK2 in health and disease: From birth to death: the role of protein kinase CK2 in the regulation of cell proliferation and survival.Cell Mol Life Sci. 2009 Jun;66(11-12):1817-29. doi: 10.1007/s00018-009-9150-2. Cell Mol Life Sci. 2009. PMID: 19387552 Free PMC article. Review.
-
Protein kinase CK2 in health and disease: Protein kinase CK2: an ugly duckling in the kinome pond.Cell Mol Life Sci. 2009 Jun;66(11-12):1795-9. doi: 10.1007/s00018-009-9148-9. Cell Mol Life Sci. 2009. PMID: 19387554 Free PMC article. Review. No abstract available.
Cited by
-
Influence of the accessory protein SET on M3 muscarinic receptor phosphorylation and G protein coupling.Mol Pharmacol. 2012 Jul;82(1):17-26. doi: 10.1124/mol.111.075523. Epub 2012 Mar 30. Mol Pharmacol. 2012. PMID: 22466417 Free PMC article.
-
N-methyl-D-aspartate receptors mediate the phosphorylation and desensitization of muscarinic receptors in cerebellar granule neurons.J Biol Chem. 2009 Jun 19;284(25):17147-17156. doi: 10.1074/jbc.M901031200. Epub 2009 Mar 30. J Biol Chem. 2009. PMID: 19332541 Free PMC article.
-
Identification of phosphorylation sites in the COOH-terminal tail of the μ-opioid receptor.J Neurochem. 2013 Jan;124(2):189-99. doi: 10.1111/jnc.12071. Epub 2012 Nov 30. J Neurochem. 2013. PMID: 23106126 Free PMC article.
-
Preassembled GPCR signaling complexes mediate distinct cellular responses to ultralow ligand concentrations.Sci Signal. 2018 Oct 9;11(551):eaan1188. doi: 10.1126/scisignal.aan1188. Sci Signal. 2018. PMID: 30301787 Free PMC article.
-
Emerging paradigms of β-arrestin-dependent seven transmembrane receptor signaling.Trends Biochem Sci. 2011 Sep;36(9):457-69. doi: 10.1016/j.tibs.2011.06.003. Epub 2011 Jul 20. Trends Biochem Sci. 2011. PMID: 21764321 Free PMC article. Review.
References
-
- Blaukat, A., A. Pizard, A. Breit, C. Wernstedt, F. Alhenc-Gelas, W. Muller-Esterl, and I. Dikic. 2001. Determination of bradykinin B2 receptor in vivo phosphorylation sites and their role in receptor function. J. Biol. Chem. 276:40431–40440. - PubMed
-
- Budd, D.C., A. Rae, and A.B. Tobin. 1999. Activation of the mitogen-activated protein kinase pathway by a Gq/11-coupled muscarinic receptor is independent of receptor internalization. J. Biol. Chem. 274:12355–12360. - PubMed
-
- Budd, D.C., J.E. McDonald, and A.B. Tobin. 2000. Phosphorylation and regulation of a Gq/11-coupled receptor by casein kinase 1alpha. J. Biol. Chem. 275:19667–19675. - PubMed
-
- Budd, D.C., G.B. Willars, J.E. McDonald, and A.B. Tobin. 2001. Phosphorylation of the Gq/11-coupled m3-muscarinic receptor is involved in receptor activation of the ERK-1/2 mitogen-activated protein kinase pathway. J. Biol. Chem. 276:4581–4587. - PubMed
-
- Budd, D.C., J. McDonald, N. Emsley, K. Cain, and A.B. Tobin. 2003. The C-terminal tail of the M3-muscarinic receptor possesses anti-apoptotic properties. J. Biol. Chem. 278:19565–19573. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

