The role of karyopherins in the regulated sumoylation of septins
- PMID: 17403926
- PMCID: PMC2064105
- DOI: 10.1083/jcb.200608066
The role of karyopherins in the regulated sumoylation of septins
Abstract
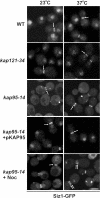
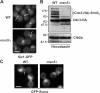
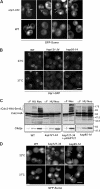
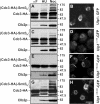
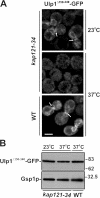
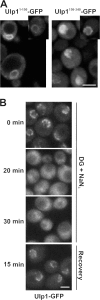
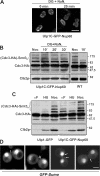
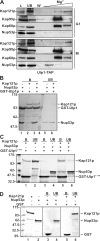
In the yeast Saccharomyces cerevisiae, several components of the septin ring are sumoylated during anaphase and then abruptly desumoylated at cytokinesis. We show that septin sumoylation is controlled by the interactions of two enzymes of the sumoylation pathway, Siz1p and Ulp1p, with the nuclear transport machinery. The E3 ligase Siz1p is imported into the nucleus by the karyopherin Kap95p during interphase. In M phase, Siz1p is exported from the nucleus by the karyopherin Kap142p/Msn5p and subsequently targeted to the septin ring, where it participates in septin sumoylation. We also show that the accumulation of sumoylated septins during mitosis is dependent on the interactions of the SUMO isopeptidase Ulp1p with Kap121p and Kap95p-Kap60p and the nuclear pore complex (NPC). In addition to sequestering Ulp1 at the NPC, Kap121p is required for targeting Ulp1p to the septin ring during mitosis. We present a model in which Ulp1p is maintained at the NPC during interphase and transiently interacts with the septin ring during mitosis.
Figures
Similar articles
-
Structures of the Karyopherins Kap121p and Kap60p Bound to the Nuclear Pore-Targeting Domain of the SUMO Protease Ulp1p.J Mol Biol. 2017 Jan 20;429(2):249-260. doi: 10.1016/j.jmb.2016.11.029. Epub 2016 Dec 6. J Mol Biol. 2017. PMID: 27939291
-
Adjunct duties for karyopherins: regulating septin sumoylation.Dev Cell. 2007 May;12(5):669-70. doi: 10.1016/j.devcel.2007.04.007. Dev Cell. 2007. PMID: 17488619
-
Sumo-dependent substrate targeting of the SUMO protease Ulp1.BMC Biol. 2011 Oct 28;9:74. doi: 10.1186/1741-7007-9-74. BMC Biol. 2011. PMID: 22034919 Free PMC article.
-
Cytoplasmic sumoylation by PIAS-type Siz1-SUMO ligase.Cell Cycle. 2008 Jun 15;7(12):1738-44. doi: 10.4161/cc.7.12.6156. Epub 2008 Jun 16. Cell Cycle. 2008. PMID: 18583943 Review.
-
SUMO and Nucleocytoplasmic Transport.Adv Exp Med Biol. 2017;963:111-126. doi: 10.1007/978-3-319-50044-7_7. Adv Exp Med Biol. 2017. PMID: 28197909 Review.
Cited by
-
Mitochondria and the culture of the Borg: understanding the integration of mitochondrial function within the reticulum, the cell, and the organism.Bioessays. 2010 Nov;32(11):958-66. doi: 10.1002/bies.201000073. Epub 2010 Sep 7. Bioessays. 2010. PMID: 20824657 Free PMC article. Review.
-
Nucleoporins prevent DNA damage accumulation by modulating Ulp1-dependent sumoylation processes.Mol Biol Cell. 2007 Aug;18(8):2912-23. doi: 10.1091/mbc.e07-02-0123. Epub 2007 May 30. Mol Biol Cell. 2007. PMID: 17538013 Free PMC article.
-
The deSUMOylase SENP2 coordinates homologous recombination and nonhomologous end joining by independent mechanisms.Genes Dev. 2019 Mar 1;33(5-6):333-347. doi: 10.1101/gad.321125.118. Epub 2019 Feb 22. Genes Dev. 2019. PMID: 30796017 Free PMC article.
-
Sumoylation regulates Kap114-mediated nuclear transport.EMBO J. 2012 May 30;31(11):2461-72. doi: 10.1038/emboj.2012.102. Epub 2012 May 4. EMBO J. 2012. PMID: 22562154 Free PMC article.
-
The SUMO-specific isopeptidase SENP2 associates dynamically with nuclear pore complexes through interactions with karyopherins and the Nup107-160 nucleoporin subcomplex.Mol Biol Cell. 2011 Dec;22(24):4868-82. doi: 10.1091/mbc.E10-12-0953. Epub 2011 Oct 26. Mol Biol Cell. 2011. PMID: 22031293 Free PMC article.
References
-
- Ghaemmaghami, S., W.K. Huh, K. Bower, R.W. Howson, A. Belle, N. Dephoure, E.K. O'Shea, and J.S. Weissman. 2003. Global analysis of protein expression in yeast. Nature. 425:737–741. - PubMed
-
- Hang, J., and M. Dasso. 2002. Association of the human SUMO-1 protease SENP2 with the nuclear pore. J. Biol. Chem. 277:19961–19966. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

