Virus glycosylation: role in virulence and immune interactions
- PMID: 17398101
- PMCID: PMC7127133
- DOI: 10.1016/j.tim.2007.03.003
Virus glycosylation: role in virulence and immune interactions
Abstract
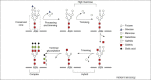
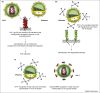
The study of N-linked glycosylation as it relates to virus biology has become an area of intense interest in recent years due to its ability to impart various advantages to virus survival and virulence. HIV and influenza, two clear threats to human health, have been shown to rely on expression of specific oligosaccharides to evade detection by the host immune system. Additionally, other viruses such as Hendra, SARS-CoV, influenza, hepatitis and West Nile rely on N-linked glycosylation for crucial functions such as entry into host cells, proteolytic processing and protein trafficking. This review focuses on recent findings on the importance of glycosylation to viral virulence and immune evasion for several prominent human pathogens.
Figures
Similar articles
-
Viral glycoproteomes: technologies for characterization and outlook for vaccine design.FEBS Lett. 2018 Dec;592(23):3898-3920. doi: 10.1002/1873-3468.13177. Epub 2018 Jul 12. FEBS Lett. 2018. PMID: 29961944 Review.
-
Cellular proteases involved in the pathogenicity of enveloped animal viruses, human immunodeficiency virus, influenza virus A and Sendai virus.Adv Enzyme Regul. 1996;36:325-47. doi: 10.1016/0065-2571(95)00016-x. Adv Enzyme Regul. 1996. PMID: 8869754
-
Glycosylation of the Hemagglutinin Protein of H5N1 Influenza Virus Increases Its Virulence in Mice by Exacerbating the Host Immune Response.J Virol. 2017 Mar 13;91(7):e02215-16. doi: 10.1128/JVI.02215-16. Print 2017 Apr 1. J Virol. 2017. PMID: 28100622 Free PMC article.
-
Glycosylation of viral proteins: Implication in virus-host interaction and virulence.Virulence. 2022 Dec;13(1):670-683. doi: 10.1080/21505594.2022.2060464. Virulence. 2022. PMID: 35436420 Free PMC article. Review.
-
Exploitation of glycosylation in enveloped virus pathobiology.Biochim Biophys Acta Gen Subj. 2019 Oct;1863(10):1480-1497. doi: 10.1016/j.bbagen.2019.05.012. Epub 2019 May 20. Biochim Biophys Acta Gen Subj. 2019. PMID: 31121217 Free PMC article. Review.
Cited by
-
Mass Spectrometry Analysis of Newly Emerging Coronavirus HCoV-19 Spike Protein and Human ACE2 Reveals Camouflaging Glycans and Unique Post-Translational Modifications.Engineering (Beijing). 2021 Oct;7(10):1441-1451. doi: 10.1016/j.eng.2020.07.014. Epub 2020 Aug 30. Engineering (Beijing). 2021. PMID: 32904601 Free PMC article.
-
Possible role of a cell surface carbohydrate in evolution of resistance to viral infections in old world primates.J Virol. 2013 Aug;87(15):8317-26. doi: 10.1128/JVI.01118-13. Epub 2013 Jun 5. J Virol. 2013. PMID: 23740988 Free PMC article.
-
The diversity of the glycan shield of sarbecoviruses closely related to SARS-CoV-2.bioRxiv [Preprint]. 2022 Aug 25:2022.08.24.505118. doi: 10.1101/2022.08.24.505118. bioRxiv. 2022. Update in: Cell Rep. 2023 Apr 25;42(4):112307. doi: 10.1016/j.celrep.2023.112307. PMID: 36052375 Free PMC article. Updated. Preprint.
-
Host Synthesized Carbohydrate Antigens on Viral Glycoproteins as "Achilles' Heel" of Viruses Contributing to Anti-Viral Immune Protection.Int J Mol Sci. 2020 Sep 13;21(18):6702. doi: 10.3390/ijms21186702. Int J Mol Sci. 2020. PMID: 32933166 Free PMC article. Review.
-
Inducing enhanced neutralizing antibodies against broad SARS-CoV-2 variants through glycan-shielding multiple non-neutralizing epitopes of RBD.Front Immunol. 2023 Dec 11;14:1259386. doi: 10.3389/fimmu.2023.1259386. eCollection 2023. Front Immunol. 2023. PMID: 38149245 Free PMC article.
References
-
- Land A., Braakman I. Folding of the human immunodeficiency virus type 1 envelope glycoprotein in the endoplasmic reticulum. Biochimie. 2001;83:783–790. - PubMed
-
- Slater-Handshy T. HCV E2 glycoprotein: mutagenesis of N-linked glycosylation sites and its effects on E2 expression and processing. Virology. 2004;319:36–48. - PubMed
-
- Meunier J.C. Analysis of the glycosylation sites of hepatitis C virus (HCV) glycoprotein E1 and the influence of E1 glycans on the formation of the HCV glycoprotein complex. J. Gen. Virol. 1999;80:887–896. - PubMed
-
- Klenk H.D. Importance of hemagglutinin glycosylation for the biological functions of influenza virus. Virus Res. 2002;82:73–75. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

