Telomere dysfunction suppresses spontaneous tumorigenesis in vivo by initiating p53-dependent cellular senescence
- PMID: 17396137
- PMCID: PMC1866197
- DOI: 10.1038/sj.embor.7400937
Telomere dysfunction suppresses spontaneous tumorigenesis in vivo by initiating p53-dependent cellular senescence
Abstract
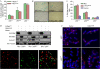
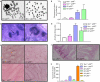
Dysfunctional telomeres induce p53-dependent cellular senescence and apoptosis, but it is not known which function is more important for tumour suppression in vivo. We used the p53 ( R172P ) knock-in mouse, which is unable to induce apoptosis but retains intact cell-cycle arrest and cellular senescence pathways, to show that spontaneous tumorigenesis is potently repressed in Terc -/- p53 ( R172P ) mice. Tumour suppression is accompanied by global induction of p53, p21 and the senescence marker senescence-associated-beta-galactosidase. By contrast, cellular senescence was unable to suppress chemically induced skin carcinomas. These results indicate that suppression of spontaneous tumorigenesis by dysfunctional telomeres requires the activation of the p53-dependent cellular senescence pathway.
Figures



Similar articles
-
p53-Dependent accelerated senescence induced by ionizing radiation in breast tumour cells.Int J Radiat Biol. 2005 Jun;81(6):445-58. doi: 10.1080/09553000500168549. Int J Radiat Biol. 2005. PMID: 16308915
-
Telomere dysfunction and tumour suppression: the senescence connection.Nat Rev Cancer. 2008 Jun;8(6):450-8. doi: 10.1038/nrc2393. Nat Rev Cancer. 2008. PMID: 18500246 Free PMC article. Review.
-
Control of Cellular Aging, Tissue Function, and Cancer by p53 Downstream of Telomeres.Cold Spring Harb Perspect Med. 2017 May 1;7(5):a026088. doi: 10.1101/cshperspect.a026088. Cold Spring Harb Perspect Med. 2017. PMID: 28289249 Free PMC article. Review.
-
Dysfunctional telomeres induce p53-dependent and independent apoptosis to compromise cellular proliferation and inhibit tumor formation.Aging Cell. 2016 Aug;15(4):646-60. doi: 10.1111/acel.12476. Epub 2016 Apr 26. Aging Cell. 2016. PMID: 27113195 Free PMC article.
-
G1 checkpoint failure and increased tumor susceptibility in mice lacking the novel p53 target Ptprv.EMBO J. 2005 Sep 7;24(17):3093-103. doi: 10.1038/sj.emboj.7600769. Epub 2005 Aug 18. EMBO J. 2005. PMID: 16107883 Free PMC article.
Cited by
-
Viral single-strand DNA induces p53-dependent apoptosis in human embryonic stem cells.PLoS One. 2011;6(11):e27520. doi: 10.1371/journal.pone.0027520. Epub 2011 Nov 17. PLoS One. 2011. PMID: 22114676 Free PMC article.
-
Telomerase directly regulates NF-κB-dependent transcription.Nat Cell Biol. 2012 Dec;14(12):1270-81. doi: 10.1038/ncb2621. Epub 2012 Nov 18. Nat Cell Biol. 2012. PMID: 23159929
-
Senescence in tumours: evidence from mice and humans.Nat Rev Cancer. 2010 Jan;10(1):51-7. doi: 10.1038/nrc2772. Nat Rev Cancer. 2010. PMID: 20029423 Free PMC article. Review.
-
Telomeric and extra-telomeric roles for telomerase and the telomere-binding proteins.Nat Rev Cancer. 2011 Mar;11(3):161-76. doi: 10.1038/nrc3025. Nat Rev Cancer. 2011. PMID: 21346783 Review.
-
PinX1: a sought-after major tumor suppressor at human chromosome 8p23.Oncotarget. 2011 Oct;2(10):810-9. doi: 10.18632/oncotarget.339. Oncotarget. 2011. PMID: 22021332 Free PMC article.
References
-
- Artandi SE, Chang S, Lee SL, Alson S, Gottlieb GJ, Chin L, DePinho RA (2000) Telomere dysfunction promotes non-reciprocal translocations and epithelial cancers in mice. Nature 406: 641–645 - PubMed
-
- Bartkova J et al. (2005) DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature 434: 864–870 - PubMed
-
- Bartkova J et al. (2006) Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature 2444: 633–637 - PubMed
-
- Blasco MA, Lee HW, Hande MP, Samper E, Lansdorp PM, DePinho RA, Greider CW (1997) Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell 91: 25–34 - PubMed
-
- Braig M, Lee S, Loddenkemper C, Rudolph C, Peters AH, Schlegelberger B, Stein H, Dorken B, Jenuwein T, Schmitt CA (2005) Oncogene-induced senescence as an initial barrier in lymphoma development. Nature 436: 660–665 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

