Three-dimensional culture models of normal and malignant breast epithelial cells
- PMID: 17396127
- PMCID: PMC2933182
- DOI: 10.1038/nmeth1015
Three-dimensional culture models of normal and malignant breast epithelial cells
Abstract
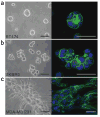
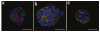
Extracellular matrix is a key regulator of normal homeostasis and tissue phenotype. Important signals are lost when cells are cultured ex vivo on two-dimensional plastic substrata. Many of these crucial microenvironmental cues may be restored using three-dimensional (3D) cultures of laminin-rich extracellular matrix (lrECM). These 3D culture assays allow phenotypic discrimination between nonmalignant and malignant mammary cells, as the former grown in a 3D context form polarized, growth-arrested acinus-like colonies whereas the latter form disorganized, proliferative and nonpolar colonies. Signaling pathways that function in parallel in cells cultured on plastic become reciprocally integrated when the cells are exposed to basement membrane-like gels. Appropriate 3D culture thus provides a more physiologically relevant approach to the analysis of gene function and cell phenotype ex vivo. We describe here a robust and generalized method for the culturing of various human breast cell lines in three dimensions and describe the preparation of cellular extracts from these cultures for molecular analyses. The procedure below describes the 3D 'embedded' assay, in which cells are cultured embedded in an lrECM gel (Fig. 1). By lrECM, we refer to the solubilized extract derived from the Engelbreth-Holm-Swarm mouse sarcoma cells. For a discussion of user options regarding 3D matrices, see Box 1. Alternatively, the 3D 'on-top' assay, in which cells are cultured on top of a thin lrECM gel overlaid with a dilute solution of lrECM, may be used as described in Box 2 (Fig. 1 and Fig. 2).
Figures

Similar articles
-
Using 3D Culture of Primary Mammary Epithelial Cells to Define Molecular Entities Required for Acinus Formation: Analyzing MAP Kinase Phosphatases.Methods Mol Biol. 2017;1501:199-216. doi: 10.1007/978-1-4939-6475-8_9. Methods Mol Biol. 2017. PMID: 27796954
-
Three-dimensional Mammary Epithelial Cell Morphogenesis Model for Analysis of TGFß Signaling.Methods Mol Biol. 2016;1344:121-35. doi: 10.1007/978-1-4939-2966-5_7. Methods Mol Biol. 2016. PMID: 26520121
-
Cell shape regulates global histone acetylation in human mammary epithelial cells.Exp Cell Res. 2007 Aug 15;313(14):3066-75. doi: 10.1016/j.yexcr.2007.04.022. Epub 2007 Apr 27. Exp Cell Res. 2007. PMID: 17524393 Free PMC article.
-
Tissue structure, nuclear organization, and gene expression in normal and malignant breast.Cancer Res. 1999 Apr 1;59(7 Suppl):1757-1763s; discussion 1763s-1764s. Cancer Res. 1999. PMID: 10197593 Review.
-
Use of three-dimensional basement membrane cultures to model oncogene-induced changes in mammary epithelial morphogenesis.J Mammary Gland Biol Neoplasia. 2004 Oct;9(4):297-310. doi: 10.1007/s10911-004-1402-z. J Mammary Gland Biol Neoplasia. 2004. PMID: 15838601 Free PMC article. Review.
Cited by
-
Exploiting the Acidic Extracellular pH: Evaluation of Streptococcus salivarius M18 Postbiotics to Target Cancer Cells.Probiotics Antimicrob Proteins. 2022 Dec;14(6):995-1011. doi: 10.1007/s12602-021-09806-3. Epub 2021 Jun 2. Probiotics Antimicrob Proteins. 2022. PMID: 34080175
-
3D porous chitosan-alginate scaffolds: a new matrix for studying prostate cancer cell-lymphocyte interactions in vitro.Adv Healthc Mater. 2012 Sep;1(5):590-9. doi: 10.1002/adhm.201100054. Epub 2012 Jul 6. Adv Healthc Mater. 2012. PMID: 23184794 Free PMC article.
-
Overexpression and β-1,6-N-acetylglucosaminylation-initiated aberrant glycosylation of TIMP-1: a "double whammy" strategy in colon cancer progression.J Biol Chem. 2012 Sep 21;287(39):32467-78. doi: 10.1074/jbc.M112.370064. Epub 2012 Aug 2. J Biol Chem. 2012. PMID: 22859303 Free PMC article.
-
Molecular profiling of prostatic acinar morphogenesis identifies PDCD4 and KLF6 as tissue architecture-specific prognostic markers in prostate cancer.Am J Pathol. 2013 Feb;182(2):363-74. doi: 10.1016/j.ajpath.2012.10.024. Epub 2012 Dec 4. Am J Pathol. 2013. PMID: 23219426 Free PMC article.
-
Microfluidics 3D gel-island chip for single cell isolation and lineage-dependent drug responses study.Lab Chip. 2016 Jul 7;16(13):2504-2512. doi: 10.1039/c6lc00081a. Epub 2016 Jun 8. Lab Chip. 2016. PMID: 27270563 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

