The DH and PH domains of Trio coordinately engage Rho GTPases for their efficient activation
- PMID: 17391702
- PMCID: PMC1890047
- DOI: 10.1016/j.jmb.2007.02.060
The DH and PH domains of Trio coordinately engage Rho GTPases for their efficient activation
Abstract
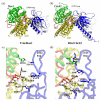
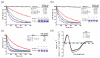
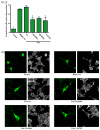
Rho-family GTPases are activated by the exchange of bound GDP for GTP, a process that is catalyzed by Dbl-family guanine nucleotide exchange factors (GEFs). The catalytic unit of Dbl-family GEFs consists of a Dbl homology (DH) domain followed almost invariantly by a pleckstrin-homology (PH) domain. The majority of the catalytic interface forms between the switch regions of the GTPase and the DH domain, but full catalytic activity often requires the associated PH domain. Although PH domains are usually characterized as lipid-binding regions, they also participate in protein-protein interactions. For example, the DH-associated PH domain of Dbs must contact its cognate GTPases for efficient exchange. Similarly, the N-terminal DH/PH fragment of Trio, which catalyzes exchange on both Rac1 and RhoG, is fourfold more active in vitro than the isolated DH domain. Given continued uncertainty regarding functional roles of DH-associated PH domains, we have undertaken structural and functional analyses of the N-terminal DH/PH cassette of Trio. The crystal structure of this fragment of Trio bound to nucleotide-depleted Rac1 highlights the engagement of the PH domain with Rac1 and substitution of residues involved in this interface substantially diminishes activation of Rac1 and RhoG. Also, these mutations significantly reduce the ability of full-length Trio to induce neurite outgrowth dependent on RhoG activation in PC-12 cells. Overall, these studies substantiate a general role for DH-associated PH domains in engaging Rho GTPases directly for efficient guanine nucleotide exchange and support a parsimonious explanation for the essentially invariant linkage between DH and PH domains.
Figures


Similar articles
-
Different regulation of the Trio Dbl-Homology domains by their associated PH domains.Biol Cell. 2003 Dec;95(9):625-34. doi: 10.1016/j.biolcel.2003.10.002. Biol Cell. 2003. PMID: 14720465
-
The Rac1- and RhoG-specific GEF domain of Trio targets filamin to remodel cytoskeletal actin.Nat Cell Biol. 2000 Dec;2(12):888-92. doi: 10.1038/35046533. Nat Cell Biol. 2000. PMID: 11146652
-
A crystallographic view of interactions between Dbs and Cdc42: PH domain-assisted guanine nucleotide exchange.EMBO J. 2002 Mar 15;21(6):1315-26. doi: 10.1093/emboj/21.6.1315. EMBO J. 2002. PMID: 11889037 Free PMC article.
-
The many faces of the guanine-nucleotide exchange factor trio.Cell Adh Migr. 2012 Nov-Dec;6(6):482-7. doi: 10.4161/cam.21418. Epub 2012 Oct 17. Cell Adh Migr. 2012. PMID: 23076143 Free PMC article. Review.
-
Structural elements, mechanism, and evolutionary convergence of Rho protein-guanine nucleotide exchange factor complexes.Biochemistry. 2004 Feb 3;43(4):837-42. doi: 10.1021/bi036026v. Biochemistry. 2004. PMID: 14744125 Review.
Cited by
-
Comparison of tertiary structures of proteins in protein-protein complexes with unbound forms suggests prevalence of allostery in signalling proteins.BMC Struct Biol. 2012 May 3;12:6. doi: 10.1186/1472-6807-12-6. BMC Struct Biol. 2012. PMID: 22554255 Free PMC article.
-
Identification and characterization of cancer mutations in Japanese lung adenocarcinoma without sequencing of normal tissue counterparts.PLoS One. 2013 Sep 12;8(9):e73484. doi: 10.1371/journal.pone.0073484. eCollection 2013. PLoS One. 2013. PMID: 24069199 Free PMC article.
-
Detection of autism spectrum disorder-related pathogenic trio variants by a novel structure-based approach.Mol Autism. 2024 Apr 3;15(1):12. doi: 10.1186/s13229-024-00590-9. Mol Autism. 2024. PMID: 38566250 Free PMC article.
-
Trio family proteins as regulators of cell migration and morphogenesis in development and disease - mechanisms and cellular contexts.J Cell Sci. 2021 Feb 10;134(3):jcs248393. doi: 10.1242/jcs.248393. J Cell Sci. 2021. PMID: 33568469 Free PMC article. Review.
-
Genome-wide DNA methylation and transcriptomic profiles in the lifestyle strategies and asexual development of the forest fungal pathogen Heterobasidion parviporum.Epigenetics. 2019 Jan;14(1):16-40. doi: 10.1080/15592294.2018.1564426. Epub 2019 Jan 19. Epigenetics. 2019. PMID: 30633603 Free PMC article.
References
-
- Hoffman GR, Cerione RA. Signaling to the Rho GTPases: networking with the DH domain. FEBS Lett. 2002;513:85–91. - PubMed
-
- Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–35. - PubMed
-
- Schwartz MA, Shattil SJ. Signaling networks linking integrins and rho family GTPases. Trends Biochem Sci. 2000;25:388–91. - PubMed
-
- Sahai E, Marshall CJ. RHO-GTPases and cancer. Nat Rev Cancer. 2002;2:133–42. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

