Involvement of interferon-gamma in microglial-mediated loss of dopaminergic neurons
- PMID: 17376993
- PMCID: PMC6672486
- DOI: 10.1523/JNEUROSCI.5321-06.2007
Involvement of interferon-gamma in microglial-mediated loss of dopaminergic neurons
Abstract
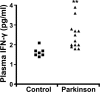
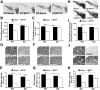
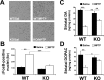
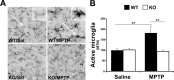
Growing evidence implicates microglia in the loss of dopaminergic neurons in Parkinson's disease (PD). However, factors mediating microglial activation in PD are poorly understood. Proinflammatory cytokines, such as interferon-gamma (IFN-gamma), orchestrate the actions of microglia. We report here that PD patients express significantly elevated levels of IFN-gamma in their blood plasma. After this initial finding, we found that IFN-gamma-deficient mice displayed attenuated 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced substantia nigra pars compacta dopaminergic cell loss along with reduced loss of striatal tyrosine hydroxylase and dopamine transporter fiber density. MPTP-induced depletion of striatal dopamine and its metabolite DOPAC (3,4-dihydroxyphenylacetic acid), as well as deltaFosB, a marker of postsynaptic dysfunction, were also attenuated in these knock-out mice. Consistent with the role for IFN-gamma in microglial activation, MPTP-induced morphological activation of microglia was abrogated compared with wild-type mice. To examine more mechanistically the role of IFN-gamma in microglial activation, we evaluated the interactions between microglia and dopaminergic neurons in an in vitro mixed microglia/midbrain neuron rotenone-induced death paradigm. In this in vitro paradigm, dopaminergic neurons are selectively damaged by rotenone. Exogenous IFN-gamma ligand alone and without rotenone resulted in dopaminergic cell loss, but only in the presence of microglia. The addition of an IFN-gamma neutralizing antibody attenuated neuronal loss as a result of rotenone treatment. The presence of only wild-type microglia and not those deficient in IFN-gamma receptor elicited significant dopaminergic cell loss when exposed to rotenone. Neurons deficient in IFN-gamma receptor, however, did not display increased resistance to death. Finally, levels of IFN-gamma message increased in microglia in response to rotenone. Together, these data suggest that IFN-gamma participates in death of dopaminergic neurons by regulating microglial activity.
Figures
Similar articles
-
Regulation of dopaminergic loss by Fas in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson's disease.J Neurosci. 2004 Feb 25;24(8):2045-53. doi: 10.1523/JNEUROSCI.4564-03.2004. J Neurosci. 2004. PMID: 14985447 Free PMC article.
-
Dopamine receptor D3 expressed on CD4+ T cells favors neurodegeneration of dopaminergic neurons during Parkinson's disease.J Immunol. 2013 May 15;190(10):5048-56. doi: 10.4049/jimmunol.1203121. Epub 2013 Apr 15. J Immunol. 2013. PMID: 23589621
-
Critical role for microglial NADPH oxidase in rotenone-induced degeneration of dopaminergic neurons.J Neurosci. 2003 Jul 16;23(15):6181-7. doi: 10.1523/JNEUROSCI.23-15-06181.2003. J Neurosci. 2003. PMID: 12867501 Free PMC article.
-
CD200 maintains the region-specific phenotype of microglia in the midbrain and its role in Parkinson's disease.Glia. 2020 Sep;68(9):1874-1890. doi: 10.1002/glia.23811. Epub 2020 Feb 29. Glia. 2020. PMID: 32112601 Review.
-
The role of glial cells in Parkinson's disease.Curr Opin Neurol. 2001 Aug;14(4):483-9. doi: 10.1097/00019052-200108000-00009. Curr Opin Neurol. 2001. PMID: 11470965 Review.
Cited by
-
Parkinson's Disease: Can Targeting Inflammation Be an Effective Neuroprotective Strategy?Front Neurosci. 2021 Feb 25;14:580311. doi: 10.3389/fnins.2020.580311. eCollection 2020. Front Neurosci. 2021. PMID: 33716638 Free PMC article. Review.
-
The Nigral Coup in Parkinson's Disease by α-Synuclein and Its Associated Rebels.Cells. 2021 Mar 9;10(3):598. doi: 10.3390/cells10030598. Cells. 2021. PMID: 33803185 Free PMC article. Review.
-
Microglial Extracellular Vesicles as Vehicles for Neurodegeneration Spreading.Biomolecules. 2021 May 21;11(6):770. doi: 10.3390/biom11060770. Biomolecules. 2021. PMID: 34063832 Free PMC article. Review.
-
Progressive dopaminergic cell loss with unilateral-to-bilateral progression in a genetic model of Parkinson disease.Proc Natl Acad Sci U S A. 2012 Sep 25;109(39):15918-23. doi: 10.1073/pnas.1205102109. Epub 2012 Sep 10. Proc Natl Acad Sci U S A. 2012. PMID: 23019375 Free PMC article.
-
Microglial cell dysregulation in brain aging and neurodegeneration.Front Aging Neurosci. 2015 Jul 20;7:124. doi: 10.3389/fnagi.2015.00124. eCollection 2015. Front Aging Neurosci. 2015. PMID: 26257642 Free PMC article. Review.
References
-
- Abbas N, Bednar I, Mix E, Marie S, Paterson D, Ljungberg A, Morris C, Winblad B, Nordberg A, Zhu J. Up-regulation of the inflammatory cytokines IFN-gamma and IL-12 and down-regulation of IL-4 in cerebral cortex regions of APP(SWE) transgenic mice. J Neuroimmunol. 2002;126:50–57. - PubMed
-
- Almer G, Vukosavic S, Romero N, Przedborski S. Inducible nitric oxide synthase up-regulation in a transgenic mouse model of familial amyotrophic lateral sclerosis. J Neurochem. 1999;72:2415–2425. - PubMed
-
- Arai H, Furuya T, Yasuda T, Miura M, Mizuno Y, Mochizuki H. Neurotoxic effects of lipopolysaccharide on nigral dopaminergic neurons are mediated by microglial activation, interleukin-1beta, and expression of caspase-11 in mice. J Biol Chem. 2004;279:51647–51653. - PubMed
-
- Badie B, Schartner J, Vorpahl J, Preston K. Interferon-gamma induces apoptosis and augments the expression of Fas and Fas ligand by microglia in vitro. Exp Neurol. 2000;162:290–296. - PubMed
-
- Banati RB, Daniel SE, Blunt SB. Glial pathology but absence of apoptotic nigral neurons in long-standing Parkinson's disease. Mov Disord. 1998;13:221–227. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
