LPS responsiveness and neutrophil chemotaxis in vivo require PMN MMP-8 activity
- PMID: 17375198
- PMCID: PMC1819564
- DOI: 10.1371/journal.pone.0000312
LPS responsiveness and neutrophil chemotaxis in vivo require PMN MMP-8 activity
Abstract
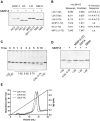
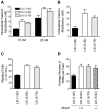
We identify matrix metalloproteinase (MMP)-8, the polymorphonuclear (PMN) leukocyte collagenase, as a critical mediator initiating lipopolysaccharide (LPS)-responsiveness in vivo. PMN infiltration towards LPS is abrogated in Mmp8-null mice. MMP-8 cleaves LPS-induced CXC chemokine (LIX) at Ser(4)-Val(5) and Lys(79)-Arg(80). LIX bioactivity is increased upon N-terminal cleavage, enhancing intracellular calcium mobilization and chemotaxis upon binding its cognate receptor, CXCR2. As there is no difference in PMN chemotaxis in Mmp8-null mice compared with wild-type mice towards synthetic analogues of MMP-8-cleaved LIX, MMP-8 is not essential for extravasation or cell migration in collagenous matrices in vivo. However, with biochemical redundancy between MMPs 1, 2, 9, and 13, which also cleave LIX at position 4 approximately 5, it was surprising to observe such a markedly reduced PMN infiltration towards LPS and LIX in Mmp8-/- mice. This lack of physiological redundancy in vivo identifies MMP-8 as a key mediator in the regulation of innate immunity. Comparable results were found with CXCL8/IL-8 and CXCL5/ENA-78, the human orthologues of LIX. MMP-8 cleaves CXCL8 at Arg(5)-Ser(6) and at Val(7)-Leu(8) in CXCL5 to activate respective chemokines. Hence, rather than collagen, these PMN chemoattractants are important MMP-8 substrates in vivo; PMN-derived MMP-8 cleaves and activates LIX to execute an in cis PMN-controlled feed-forward mechanism to orchestrate the initial inflammatory response and promote LPS responsiveness in tissue.
Conflict of interest statement
Figures
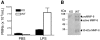

Similar articles
-
Gelatinase B/MMP-9 and neutrophil collagenase/MMP-8 process the chemokines human GCP-2/CXCL6, ENA-78/CXCL5 and mouse GCP-2/LIX and modulate their physiological activities.Eur J Biochem. 2003 Sep;270(18):3739-49. doi: 10.1046/j.1432-1033.2003.03760.x. Eur J Biochem. 2003. PMID: 12950257
-
Matrix metalloproteinase-8 facilitates neutrophil migration through the corneal stromal matrix by collagen degradation and production of the chemotactic peptide Pro-Gly-Pro.Am J Pathol. 2008 Jul;173(1):144-53. doi: 10.2353/ajpath.2008.080081. Epub 2008 Jun 13. Am J Pathol. 2008. PMID: 18556780 Free PMC article.
-
Membrane-bound matrix metalloproteinase-8 on activated polymorphonuclear cells is a potent, tissue inhibitor of metalloproteinase-resistant collagenase and serpinase.J Immunol. 2004 Jun 15;172(12):7791-803. doi: 10.4049/jimmunol.172.12.7791. J Immunol. 2004. PMID: 15187163
-
Sequence similarities of a subgroup of CXC chemokines related to murine LIX: implications for the interpretation of evolutionary relationships among chemokines.J Leukoc Biol. 1997 Nov;62(5):598-603. doi: 10.1002/jlb.62.5.598. J Leukoc Biol. 1997. PMID: 9365114 Review.
-
Neutrophil gelatinase B and chemokines in leukocytosis and stem cell mobilization.Leuk Lymphoma. 2002 Feb;43(2):233-41. doi: 10.1080/10428190290005982. Leuk Lymphoma. 2002. PMID: 11999552 Review.
Cited by
-
Proteoglycans: key regulators of pulmonary inflammation and the innate immune response to lung infection.Anat Rec (Hoboken). 2010 Jun;293(6):968-81. doi: 10.1002/ar.21094. Anat Rec (Hoboken). 2010. PMID: 20503391 Free PMC article. Review.
-
Metalloproteinases and their natural inhibitors in inflammation and immunity.Nat Rev Immunol. 2013 Sep;13(9):649-65. doi: 10.1038/nri3499. Nat Rev Immunol. 2013. PMID: 23969736 Review.
-
Increased GM-CSF-producing NCR- ILC3s and neutrophils in the intestinal mucosa exacerbate inflammatory bowel disease.Clin Transl Immunology. 2021 Jul 8;10(7):e1311. doi: 10.1002/cti2.1311. eCollection 2021. Clin Transl Immunology. 2021. PMID: 34262760 Free PMC article.
-
Biochemical characterization and N-terminomics analysis of leukolysin, the membrane-type 6 matrix metalloprotease (MMP25): chemokine and vimentin cleavages enhance cell migration and macrophage phagocytic activities.J Biol Chem. 2012 Apr 13;287(16):13382-95. doi: 10.1074/jbc.M111.314179. Epub 2012 Feb 24. J Biol Chem. 2012. PMID: 22367194 Free PMC article.
-
Influence of obesity on remodeling of lung tissue and organization of extracellular matrix after blunt thorax trauma.Respir Res. 2020 Sep 17;21(1):238. doi: 10.1186/s12931-020-01502-0. Respir Res. 2020. PMID: 32943048 Free PMC article.
References
-
- Faurschou M, Borregaard N. Neutrophil granules and secretory vesicles in inflammation. Microbes Infect. 2003;5:1317–1327. - PubMed
-
- Park JE, Barbul A. Understanding the role of immune regulation in wound healing. Am J Surg. 2004;187:11S–16S. - PubMed
-
- Bodey GP, Buckley M, Sathe YS, Freireich EJ. Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Ann Intern Med. 1966;64:328–340. - PubMed
-
- Pizzo PA. Management of fever in patients with cancer and treatment-induced neutropenia. N Engl J Med. 1993;328:1323–1332. - PubMed
-
- Hughes WT, Armstrong D, Bodey GP, Bow EJ, Brown AE, et al. 2002 guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Clin Infect Dis. 2002;34:730–751. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

