Regulation of dev, an operon that includes genes essential for Myxococcus xanthus development and CRISPR-associated genes and repeats
- PMID: 17369305
- PMCID: PMC1913320
- DOI: 10.1128/JB.00187-07
Regulation of dev, an operon that includes genes essential for Myxococcus xanthus development and CRISPR-associated genes and repeats
Abstract
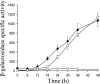
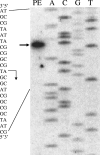
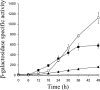
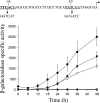
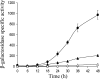
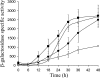
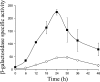
Expression of dev genes is important for triggering spore differentiation inside Myxococcus xanthus fruiting bodies. DNA sequence analysis suggested that dev and cas (CRISPR-associated) genes are cotranscribed at the dev locus, which is adjacent to CRISPR (clustered regularly interspaced short palindromic repeats). Analysis of RNA from developing M. xanthus confirmed that dev and cas genes are cotranscribed with a short upstream gene and at least two repeats of the downstream CRISPR, forming the dev operon. The operon is subject to strong, negative autoregulation during development by DevS. The dev promoter was identified. Its -35 and -10 regions resemble those recognized by M. xanthus sigma(A) RNA polymerase, the homolog of Escherichia coli sigma(70), but the spacer may be too long (20 bp); there is very little expression during growth. Induction during development relies on at least two positive regulatory elements located in the coding region of the next gene upstream. At least two positive regulatory elements and one negative element lie downstream of the dev promoter, such that the region controlling dev expression spans more than 1 kb. The results of testing different fragments for dev promoter activity in wild-type and devS mutant backgrounds strongly suggest that upstream and downstream regulatory elements interact functionally. Strikingly, the 37-bp sequence between the two CRISPR repeats that, minimally, are cotranscribed with dev and cas genes exactly matches a sequence in the bacteriophage Mx8 intP gene, which encodes a form of the integrase needed for lysogenization of M. xanthus.
Figures

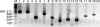

Similar articles
-
The dev Operon Regulates the Timing of Sporulation during Myxococcus xanthus Development.J Bacteriol. 2017 Apr 25;199(10):e00788-16. doi: 10.1128/JB.00788-16. Print 2017 May 15. J Bacteriol. 2017. PMID: 28264995 Free PMC article.
-
devI is an evolutionarily young negative regulator of Myxococcus xanthus development.J Bacteriol. 2015 Apr;197(7):1249-62. doi: 10.1128/JB.02542-14. Epub 2015 Feb 2. J Bacteriol. 2015. PMID: 25645563 Free PMC article.
-
devRS, an autoregulated and essential genetic locus for fruiting body development in Myxococcus xanthus.J Bacteriol. 1993 Nov;175(22):7450-62. doi: 10.1128/jb.175.22.7450-7462.1993. J Bacteriol. 1993. PMID: 7693658 Free PMC article.
-
Highly Signal-Responsive Gene Regulatory Network Governing Myxococcus Development.Trends Genet. 2017 Jan;33(1):3-15. doi: 10.1016/j.tig.2016.10.006. Epub 2016 Dec 2. Trends Genet. 2017. PMID: 27916428 Free PMC article. Review.
-
The complex global response to copper in the multicellular bacterium Myxococcus xanthus.Metallomics. 2018 Jul 18;10(7):876-886. doi: 10.1039/c8mt00121a. Metallomics. 2018. PMID: 29961779 Review.
Cited by
-
I can see CRISPR now, even when phage are gone: a view on alternative CRISPR-Cas functions from the prokaryotic envelope.Curr Opin Infect Dis. 2015 Jun;28(3):267-74. doi: 10.1097/QCO.0000000000000154. Curr Opin Infect Dis. 2015. PMID: 25887612 Free PMC article. Review.
-
EspA, an orphan hybrid histidine protein kinase, regulates the timing of expression of key developmental proteins of Myxococcus xanthus.J Bacteriol. 2008 Jul;190(13):4416-26. doi: 10.1128/JB.00265-08. Epub 2008 Apr 4. J Bacteriol. 2008. PMID: 18390653 Free PMC article.
-
Fatty acids from membrane lipids become incorporated into lipid bodies during Myxococcus xanthus differentiation.PLoS One. 2014 Jun 6;9(6):e99622. doi: 10.1371/journal.pone.0099622. eCollection 2014. PLoS One. 2014. PMID: 24906161 Free PMC article.
-
The CRISPR-Cas9 system in Neisseria spp.Pathog Dis. 2017 Jun 1;75(4):ftx036. doi: 10.1093/femspd/ftx036. Pathog Dis. 2017. PMID: 28369433 Free PMC article. Review.
-
CRISPR-Cas adaptive immunity and the three Rs.Biosci Rep. 2017 Jul 16;37(4):BSR20160297. doi: 10.1042/BSR20160297. Print 2017 Aug 31. Biosci Rep. 2017. PMID: 28674106 Free PMC article.
References
-
- Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. - PubMed
-
- Ansari, A. Z., J. E. Bradner, and T. V. O'Halloran. 1995. DNA-bend modulation in a repressor-to-activator switching mechanism. Nature 374:371-375. - PubMed
-
- Arnosti, D. N., and M. M. Kulkarni. 2005. Transcriptional enhancers: intelligent enhanceosomes or flexible billboards? J. Cell. Biochem. 94:890-898. - PubMed
-
- Biran, D., and L. Kroos. 1997. In vitro transcription of Myxococcus xanthus genes with RNA polymerase containing σA, the major sigma factor in growing cells. Mol. Microbiol. 25:463-472. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

