Effect of vascular normalization by antiangiogenic therapy on interstitial hypertension, peritumor edema, and lymphatic metastasis: insights from a mathematical model
- PMID: 17363594
- PMCID: PMC3022341
- DOI: 10.1158/0008-5472.CAN-06-4102
Effect of vascular normalization by antiangiogenic therapy on interstitial hypertension, peritumor edema, and lymphatic metastasis: insights from a mathematical model
Abstract
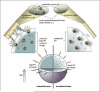
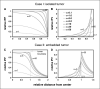
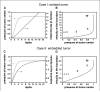
Preclinical and clinical evidence shows that antiangiogenic agents can decrease tumor vessel permeability and interstitial fluid pressure (IFP) in a process of vessel "normalization." The resulting normalized vasculature has more efficient perfusion, but little is known about how tumor IFP and interstitial fluid velocity (IFV) are affected by changes in transport properties of the vessels and interstitium that are associated with antiangiogenic therapy. By using a mathematical model to simulate IFP and IFV profiles in tumors, we show here that antiangiogenic therapy can decrease IFP by decreasing the tumor size, vascular hydraulic permeability, and/or the surface area per unit tissue volume of tumor vessels. Within a certain window of antiangiogenic effects, interstitial convection within the tumor can increase dramatically, whereas fluid convection out of the tumor margin decreases. This would result in increased drug convection within the tumor and decreased convection of drugs, growth factors, or metastatic cancer cells from the tumor margin into the peritumor fluid or tissue. Decreased convection of growth factors, such as vascular endothelial growth factor-C (VEGF-C), would limit peritumor hyperplasia, and decreased VEGF-A would limit angiogenesis in sentinel lymph nodes. Both of these effects would reduce the probability of lymphatic metastasis. Finally, decreased fluid convection into the peritumor tissue would decrease peritumor edema associated with brain tumors and ascites accumulation in the peritoneal or pleural cavity, a major complication with a number of malignancies.
Figures
Similar articles
-
Overexpression of vascular endothelial growth factor 165 drives peritumor interstitial convection and induces lymphatic drain: magnetic resonance imaging, confocal microscopy, and histological tracking of triple-labeled albumin.Cancer Res. 2002 Nov 15;62(22):6731-9. Cancer Res. 2002. PMID: 12438274
-
The effect of interstitial pressure on tumor growth: coupling with the blood and lymphatic vascular systems.J Theor Biol. 2013 Mar 7;320:131-51. doi: 10.1016/j.jtbi.2012.11.031. Epub 2012 Dec 7. J Theor Biol. 2013. PMID: 23220211 Free PMC article.
-
The effect of interstitial pressure on therapeutic agent transport: coupling with the tumor blood and lymphatic vascular systems.J Theor Biol. 2014 Aug 21;355:194-207. doi: 10.1016/j.jtbi.2014.04.012. Epub 2014 Apr 19. J Theor Biol. 2014. PMID: 24751927 Free PMC article.
-
Molecular control of lymphatic metastasis.Ann N Y Acad Sci. 2008;1131:225-34. doi: 10.1196/annals.1413.020. Ann N Y Acad Sci. 2008. PMID: 18519975 Review.
-
Role of the VEGF/VEGFR axis in cancer biology and therapy.Adv Cancer Res. 2012;114:237-67. doi: 10.1016/B978-0-12-386503-8.00006-5. Adv Cancer Res. 2012. PMID: 22588059 Review.
Cited by
-
Effect of fluid friction on interstitial fluid flow coupled with blood flow through solid tumor microvascular network.Comput Math Methods Med. 2015;2015:673426. doi: 10.1155/2015/673426. Epub 2015 Apr 19. Comput Math Methods Med. 2015. PMID: 25960764 Free PMC article.
-
Vascular endothelial growth factor inhibitors in malignant gliomas.Target Oncol. 2010 Sep;5(3):167-74. doi: 10.1007/s11523-010-0158-1. Epub 2010 Sep 7. Target Oncol. 2010. PMID: 20821351 Review.
-
Assessment of perfusion MRI-derived parameters in evaluating and predicting response to antiangiogenic therapy in patients with newly diagnosed glioblastoma.Neuro Oncol. 2011 Jan;13(1):119-31. doi: 10.1093/neuonc/noq143. Epub 2010 Oct 29. Neuro Oncol. 2011. PMID: 21036812 Free PMC article.
-
TSU68, an antiangiogenic receptor tyrosine kinase inhibitor, induces tumor vascular normalization in a human cancer xenograft nude mouse model.Surg Today. 2009;39(12):1046-53. doi: 10.1007/s00595-009-4020-y. Epub 2009 Dec 8. Surg Today. 2009. PMID: 19997799
-
Prior anti-CAFs break down the CAFs barrier and improve accumulation of docetaxel micelles in tumor.Int J Nanomedicine. 2018 Oct 4;13:5971-5990. doi: 10.2147/IJN.S171224. eCollection 2018. Int J Nanomedicine. 2018. PMID: 30323586 Free PMC article.
References
-
- Jain RK. Barriers to drug delivery in solid tumors. Sci Am. 1994;271:58–65. - PubMed
-
- Jain RK. Transport of molecules in the tumor interstitium: a review. Cancer Res. 1987;47:3038–50. - PubMed
-
- Jain RK. Transport of molecules across tumor vasculature. Cancer Metastasis Rev. 1987;6:559–94. - PubMed
-
- Boucher Y, Jain RK. Microvascular pressure is the principal driving force for interstitial hypertension in solid tumors: implications for vascular collapse. Cancer Res. 1992;52:5110–4. - PubMed
-
- Boucher Y, Leunig M, Jain RK. Tumor angiogenesis and interstitial hypertension. Cancer Res. 1996;56:4264–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

