Silencing of neuroligin function by postsynaptic neurexins
- PMID: 17360903
- PMCID: PMC2839889
- DOI: 10.1523/JNEUROSCI.0032-07.2007
Silencing of neuroligin function by postsynaptic neurexins
Abstract
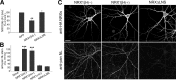
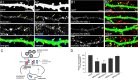
The formation of neuronal circuits during development involves a combination of synapse stabilization and elimination events. Synaptic adhesion molecules are thought to play an important role in synaptogenesis, and several trans-synaptic adhesion systems that promote the formation and maturation of synapses have been identified. The neuroligin-neurexin complex is a heterophilic adhesion system that promotes assembly and maturation of synapses through bidirectional signaling. In this protein complex, postsynaptic neuroligins are thought to interact trans-synaptically with presynaptic neurexins. However, the subcellular localization of neurexins has not been determined. Using immunoelectron microscopy, we found that endogenous neurexins and epitope-tagged neurexin-1beta are localized to axons and presynaptic terminals in vivo. Unexpectedly, neurexins are also abundant in the postsynaptic density. cis-expression of neurexin-1beta with neuroligin-1 inhibits trans-binding to recombinant neurexins, blocks the synaptogenic activity of neuroligin-1, and reduces the density of presynaptic terminals in cultured hippocampal neurons. Our results demonstrate that the function of neurexin proteins is more diverse than previously anticipated and suggest that postsynaptic cis-interactions might provide a novel mechanism for silencing the activity of a synaptic adhesion complex.
Figures
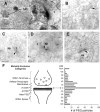
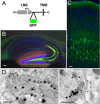
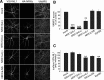
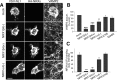
Similar articles
-
A splice code for trans-synaptic cell adhesion mediated by binding of neuroligin 1 to alpha- and beta-neurexins.Neuron. 2005 Oct 20;48(2):229-36. doi: 10.1016/j.neuron.2005.08.026. Neuron. 2005. PMID: 16242404
-
Neurexin mediates the assembly of presynaptic terminals.Nat Neurosci. 2003 Jul;6(7):708-16. doi: 10.1038/nn1074. Nat Neurosci. 2003. PMID: 12796785 Free PMC article.
-
SPARCL1 Promotes Excitatory But Not Inhibitory Synapse Formation and Function Independent of Neurexins and Neuroligins.J Neurosci. 2020 Oct 14;40(42):8088-8102. doi: 10.1523/JNEUROSCI.0454-20.2020. Epub 2020 Sep 24. J Neurosci. 2020. PMID: 32973045 Free PMC article.
-
Progress from the postsynaptic side: signaling in synaptic differentiation.Sci STKE. 2005 Mar 8;2005(274):pe9. doi: 10.1126/stke.2742005pe9. Sci STKE. 2005. PMID: 15755927 Review.
-
A matter of balance: role of neurexin and neuroligin at the synapse.Neurochem Res. 2013 Jun;38(6):1174-89. doi: 10.1007/s11064-013-1029-9. Epub 2013 Apr 5. Neurochem Res. 2013. PMID: 23559421 Review.
Cited by
-
Cell adhesion molecules regulating astrocyte-neuron interactions.Curr Opin Neurobiol. 2021 Aug;69:170-177. doi: 10.1016/j.conb.2021.03.015. Epub 2021 May 3. Curr Opin Neurobiol. 2021. PMID: 33957433 Free PMC article. Review.
-
Building and remodeling synapses.Hippocampus. 2012 May;22(5):954-68. doi: 10.1002/hipo.20872. Epub 2010 Sep 29. Hippocampus. 2012. PMID: 20882551 Free PMC article.
-
Neuronal cell type-specific alternative splicing is regulated by the KH domain protein SLM1.J Cell Biol. 2014 Feb 3;204(3):331-42. doi: 10.1083/jcb.201310136. Epub 2014 Jan 27. J Cell Biol. 2014. PMID: 24469635 Free PMC article.
-
Presenilin/γ-secretase regulates neurexin processing at synapses.PLoS One. 2011 Apr 29;6(4):e19430. doi: 10.1371/journal.pone.0019430. PLoS One. 2011. PMID: 21559374 Free PMC article.
-
Interaction between autism-linked MDGAs and neuroligins suppresses inhibitory synapse development.J Cell Biol. 2013 Feb 4;200(3):321-36. doi: 10.1083/jcb.201206028. Epub 2013 Jan 28. J Cell Biol. 2013. PMID: 23358245 Free PMC article.
References
-
- Aoki C, Miko I, Oviedo H, Mikeladze-Dvali T, Alexandre L, Sweeney N, Bredt DS. Electron microscopic immunocytochemical detection of PSD-95, PSD-93, SAP-102, and SAP-97 at postsynaptic, presynaptic, and nonsynaptic sites of adult and neonatal rat visual cortex. Synapse. 2001;40:239–257. - PubMed
-
- Aoki C, Sekino Y, Hanamura K, Fujisawa S, Mahadomrongkul V, Ren Y, Shirao T. Drebrin A is a postsynaptic protein that localizes in vivo to the submembranous surface of dendritic sites forming excitatory synapses. J Comp Neurol. 2005;483:383–402. - PubMed
-
- Benson DL, Colman DR, Huntley GW. Molecules, maps and synapse specificity. Nat Rev Neurosci. 2001;2:899–909. - PubMed
-
- Biederer T, Scheiffele P. Mixed-culture assays for analyzing neuronal synapse formation. Nat Protoc. 2007 in press. - PubMed
-
- Boucard AA, Chubykin AA, Comoletti D, Taylor P, Südhof TC. A splice code for trans-synaptic cell adhesion mediated by binding of neuroligin 1 to alpha- and beta-neurexins. Neuron. 2005;48:229–236. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

