The pRb/E2F cell-cycle pathway mediates cell death in Parkinson's disease
- PMID: 17360686
- PMCID: PMC1805567
- DOI: 10.1073/pnas.0611671104
The pRb/E2F cell-cycle pathway mediates cell death in Parkinson's disease
Abstract
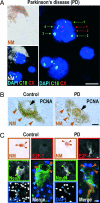
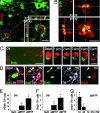
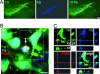
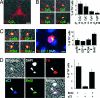
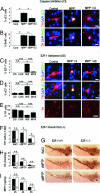
The mechanisms leading to degeneration of dopaminergic neurons (DNs) in the substantia nigra of patients with Parkinson's disease (PD) are not completely understood. Here, we show, in the postmortem human tissue, that these neurons aberrantly express mitosis-associated proteins, including the E2F-1 transcription factor, and appear to duplicate their nuclear DNA. We further demonstrate that the dopaminergic neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine injected into mice and application of its active metabolite 1-methyl-4-phenylpyridinium to mesencephalic cultures activate the retinoblastoma-E2F pathway in postmitotic DNs. We also find that cell death rather than mitotic division followed the toxin-induced replication of DNA, as determined by BrdU incorporation in DNs. In addition, blocking E2F-1 transcription protected cultured DNs against 1-methyl-4-phenylpyridinium toxicity. Finally, E2F-1-deficient mice were significantly more resistant to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced dopaminergic cell death than their wild-type littermates. Altogether, BrdU incorporation in mature neurons and lack of evidence for newborn neurons argue against neuronal turnover in normal conditions or during pathological states in the substantia nigra. Instead, our results demonstrate that mitosis-like signals are activated in mature DNs in patients with PD and mediate neuronal death in experimental models of the disease. Inhibition of mitosis-like signals may therefore provide strategies for neuroprotection in PD.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Early signs of neuronal apoptosis in the substantia nigra pars compacta of the progressive neurodegenerative mouse 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine/probenecid model of Parkinson's disease.Neuroscience. 2006 Jun 19;140(1):67-76. doi: 10.1016/j.neuroscience.2006.02.007. Epub 2006 Mar 14. Neuroscience. 2006. PMID: 16533572
-
Acetylcholinesterase deficiency decreases apoptosis in dopaminergic neurons in the neurotoxin model of Parkinson's disease.Int J Biochem Cell Biol. 2013 Feb;45(2):265-72. doi: 10.1016/j.biocel.2012.11.015. Epub 2012 Nov 29. Int J Biochem Cell Biol. 2013. PMID: 23201480
-
Systemically administered neuregulin-1β1 rescues nigral dopaminergic neurons via the ErbB4 receptor tyrosine kinase in MPTP mouse models of Parkinson's disease.J Neurochem. 2015 May;133(4):590-7. doi: 10.1111/jnc.13026. Epub 2015 Jan 26. J Neurochem. 2015. PMID: 25581060
-
Programmed cell death: does it play a role in Parkinson's disease?Ann Neurol. 1998 Sep;44(3 Suppl 1):S126-33. doi: 10.1002/ana.410440719. Ann Neurol. 1998. PMID: 9749584 Review.
-
Tp53 gene mediates distinct dopaminergic neuronal damage in different dopaminergic neurotoxicant models.Neural Regen Res. 2017 Sep;12(9):1413-1417. doi: 10.4103/1673-5374.215243. Neural Regen Res. 2017. PMID: 29089978 Free PMC article. Review.
Cited by
-
Scratch2 prevents cell cycle re-entry by repressing miR-25 in postmitotic primary neurons.J Neurosci. 2013 Mar 20;33(12):5095-105. doi: 10.1523/JNEUROSCI.4459-12.2013. J Neurosci. 2013. PMID: 23516276 Free PMC article.
-
Retinal degeneration depends on Bmi1 function and reactivation of cell cycle proteins.Proc Natl Acad Sci U S A. 2013 Feb 12;110(7):E593-601. doi: 10.1073/pnas.1108297110. Epub 2013 Jan 28. Proc Natl Acad Sci U S A. 2013. PMID: 23359713 Free PMC article.
-
Neuronal cell cycle: the neuron itself and its circumstances.Cell Cycle. 2015;14(5):712-20. doi: 10.1080/15384101.2015.1004937. Cell Cycle. 2015. PMID: 25590687 Free PMC article. Review.
-
p38 phosphorylates Rb on Ser567 by a novel, cell cycle-independent mechanism that triggers Rb-Hdm2 interaction and apoptosis.Oncogene. 2011 Feb 3;30(5):588-99. doi: 10.1038/onc.2010.442. Epub 2010 Sep 27. Oncogene. 2011. PMID: 20871633 Free PMC article.
-
Cdk5 nuclear localization is p27-dependent in nerve cells: implications for cell cycle suppression and caspase-3 activation.J Biol Chem. 2010 Apr 30;285(18):14052-61. doi: 10.1074/jbc.M109.068262. Epub 2010 Feb 26. J Biol Chem. 2010. PMID: 20189989 Free PMC article.
References
-
- Dauer W, Przedborski S. Neuron. 2003;39:889–909. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

