Regulation of brefeldin A-inhibited guanine nucleotide-exchange protein 1 (BIG1) and BIG2 activity via PKA and protein phosphatase 1gamma
- PMID: 17360629
- PMCID: PMC1805514
- DOI: 10.1073/pnas.0611696104
Regulation of brefeldin A-inhibited guanine nucleotide-exchange protein 1 (BIG1) and BIG2 activity via PKA and protein phosphatase 1gamma
Abstract
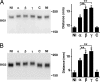
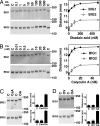
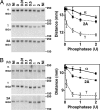
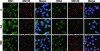
Brefeldin A-inhibited guanine nucleotide-exchange proteins (GEPs) BIG1 and BIG2 activate ADP-ribosylation factor (ARF) GTPases, which are required for vesicular trafficking. Both molecules contain one or more sites for binding protein kinase A, i.e., A kinase-anchoring protein (AKAP) sequences. Elevation of cell cAMP caused PKA-catalyzed phosphorylation and nuclear accumulation of BIG1 but not BIG2. We then asked whether BIG1 phosphorylation altered its GEP activity. Incubation of BIG1 or BIG2 with PKA catalytic subunits and ATP resulted in retardation of their electrophoretic migration, consistent with PKA phosphorylation. Okadaic acid inhibits many protein phosphatases, including protein phosphatase 1 (PP1) and PP2A, that can reverse PKA-catalyzed phosphorylation. Incubation of HepG2 cells with okadaic acid caused concentration-dependent accumulation of presumably phosphorylated BIG1 and BIG2 with decreased mobility, which was increased by subsequent incubation in vitro with specific recombinant phosphatases, PP1gamma > PP2A >> PP1alpha. For assays of GEP activity, BIG1 and BIG2 were immunoprecipitated from cells that had been depleted, respectively, of BIG2 and BIG1 by using specific siRNA. GEP activity of each was significantly decreased after incubation with recombinant PKA plus ATP and restored by incubation with PP1gamma. In agreement with a role for PP1gamma in regulation of BIG, endogenous PP1gamma, but not PP1alpha or beta, was immunoprecipitated with BIG1 or BIG2 from microsomal fractions. All observations are consistent with the effects of BIG1 and BIG2 phosphorylation on vesicular trafficking, via alterations in ARF activation and regulatory roles for cAMP, PKA, and PP1gamma in ARF activation by BIG1 and BIG2.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

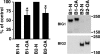
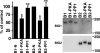

Similar articles
-
Enhancement of β-catenin activity by BIG1 plus BIG2 via Arf activation and cAMP signals.Proc Natl Acad Sci U S A. 2016 May 24;113(21):5946-51. doi: 10.1073/pnas.1601918113. Epub 2016 May 9. Proc Natl Acad Sci U S A. 2016. PMID: 27162341 Free PMC article.
-
Interaction of phosphodiesterase 3A with brefeldin A-inhibited guanine nucleotide-exchange proteins BIG1 and BIG2 and effect on ARF1 activity.Proc Natl Acad Sci U S A. 2009 Apr 14;106(15):6158-63. doi: 10.1073/pnas.0901558106. Epub 2009 Mar 30. Proc Natl Acad Sci U S A. 2009. PMID: 19332778 Free PMC article.
-
BIG1 and BIG2, brefeldin A-inhibited guanine nucleotide-exchange factors for ADP-ribosylation factors.Methods Enzymol. 2005;404:174-84. doi: 10.1016/S0076-6879(05)04017-6. Methods Enzymol. 2005. PMID: 16413268
-
Regulating the regulators: role of phosphorylation in modulating the function of the GBF1/BIG family of Sec7 ARF-GEFs.FEBS Lett. 2020 Jul;594(14):2213-2226. doi: 10.1002/1873-3468.13798. Epub 2020 May 14. FEBS Lett. 2020. PMID: 32333796 Review.
-
Allosteric regulation of Arf GTPases and their GEFs at the membrane interface.Small GTPases. 2016 Oct;7(4):283-296. doi: 10.1080/21541248.2016.1215778. Epub 2016 Jul 22. Small GTPases. 2016. PMID: 27449855 Free PMC article. Review.
Cited by
-
GENE EXPRESSION PROFILING OF HUMAN LIVER CARCINOMA (HepG2) CELLS EXPOSED TO THE MARINE TOXIN OKADAIC ACID.Toxicol Environ Chem. 2012;24(9):1805-1821. doi: 10.1080/02772248.2012.730199. Epub 2012 Oct 10. Toxicol Environ Chem. 2012. PMID: 23172983 Free PMC article.
-
Novel C-terminal motif within Sec7 domain of guanine nucleotide exchange factors regulates ADP-ribosylation factor (ARF) binding and activation.J Biol Chem. 2011 Oct 21;286(42):36898-906. doi: 10.1074/jbc.M111.230631. Epub 2011 Aug 2. J Biol Chem. 2011. PMID: 21828055 Free PMC article.
-
Regulating the large Sec7 ARF guanine nucleotide exchange factors: the when, where and how of activation.Cell Mol Life Sci. 2014 Sep;71(18):3419-38. doi: 10.1007/s00018-014-1602-7. Epub 2014 Apr 13. Cell Mol Life Sci. 2014. PMID: 24728583 Free PMC article. Review.
-
IGF-1 drives chromogranin A secretion via activation of Arf1 in human neuroendocrine tumour cells.J Cell Mol Med. 2015 May;19(5):948-59. doi: 10.1111/jcmm.12473. Epub 2015 Mar 8. J Cell Mol Med. 2015. PMID: 25754106 Free PMC article.
-
Directing Traffic: Regulation of COPI Transport by Post-translational Modifications.Front Cell Dev Biol. 2019 Sep 11;7:190. doi: 10.3389/fcell.2019.00190. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31572722 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

