C-phycocyanin hydration water dynamics in the presence of trehalose: an incoherent elastic neutron scattering study at different energy resolutions
- PMID: 17350998
- PMCID: PMC1868977
- DOI: 10.1529/biophysj.106.092114
C-phycocyanin hydration water dynamics in the presence of trehalose: an incoherent elastic neutron scattering study at different energy resolutions
Abstract
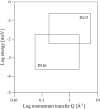
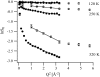
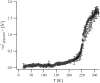
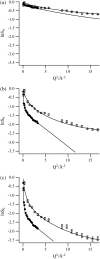
We present a study of C-phycocyanin hydration water dynamics in the presence of trehalose by incoherent elastic neutron scattering. By combining data from two backscattering spectrometers with a 10-fold difference in energy resolution we extract a scattering law S(Q,omega) from the Q-dependence of the elastic intensities without sampling the quasielastic range. The hydration water is described by two dynamically different populations--one diffusing inside a sphere and the other diffusing quasifreely--with a population ratio that depends on temperature. The scattering law derived describes the experimental data from both instruments excellently over a large temperature range (235-320 K). The effective diffusion coefficient extracted is reduced by a factor of 10-15 with respect to bulk water at corresponding temperatures. Our approach demonstrates the benefits and the efficiency of using different energy resolutions in incoherent elastic neutron scattering over a large angular range for the study of biological macromolecules and hydration water.
Figures
Similar articles
-
Dynamics of C-phycocyanin in various deuterated trehalose/water environments measured by quasielastic and elastic neutron scattering.Eur Biophys J. 2008 Jul;37(6):739-48. doi: 10.1007/s00249-007-0248-x. Epub 2008 Jan 8. Eur Biophys J. 2008. PMID: 18185929 Free PMC article.
-
Further evidence that interfacial water is the main "driving force" of protein dynamics: a neutron scattering study on perdeuterated C-phycocyanin.Phys Chem Chem Phys. 2012 Apr 14;14(14):4927-34. doi: 10.1039/c2cp23725c. Epub 2012 Mar 5. Phys Chem Chem Phys. 2012. PMID: 22388956
-
Using polarization analysis to separate the coherent and incoherent scattering from protein samples.Biochim Biophys Acta. 2010 Jan;1804(1):76-82. doi: 10.1016/j.bbapap.2009.06.024. Epub 2009 Jul 10. Biochim Biophys Acta. 2010. PMID: 19595800
-
Protein dynamics investigated by neutron scattering.Photosynth Res. 2009 Nov-Dec;102(2-3):281-93. doi: 10.1007/s11120-009-9480-9. Epub 2009 Sep 11. Photosynth Res. 2009. PMID: 19763874 Review.
-
Incoherent Neutron Scattering and Terahertz Time-Domain Spectroscopy on Protein and Hydration Water.Life (Basel). 2023 Jan 23;13(2):318. doi: 10.3390/life13020318. Life (Basel). 2023. PMID: 36836676 Free PMC article. Review.
Cited by
-
Differences in internal dynamics of actin under different structural states detected by neutron scattering.Biophys J. 2008 Jun;94(12):4880-9. doi: 10.1529/biophysj.107.125302. Epub 2008 Mar 7. Biophys J. 2008. PMID: 18326640 Free PMC article.
-
Dynamics of apomyoglobin in the alpha-to-beta transition and of partially unfolded aggregated protein.Eur Biophys J. 2009 Feb;38(2):237-44. doi: 10.1007/s00249-008-0375-z. Epub 2008 Oct 14. Eur Biophys J. 2009. PMID: 18853152
-
Bio-protective effects of homologous disaccharides on biological macromolecules.Eur Biophys J. 2012 Apr;41(4):361-7. doi: 10.1007/s00249-011-0760-x. Epub 2011 Oct 30. Eur Biophys J. 2012. PMID: 22038121
-
Protein dynamics and stability: the distribution of atomic fluctuations in thermophilic and mesophilic dihydrofolate reductase derived using elastic incoherent neutron scattering.Biophys J. 2008 Jun;94(12):4812-8. doi: 10.1529/biophysj.107.121418. Epub 2008 Feb 29. Biophys J. 2008. PMID: 18310248 Free PMC article.
-
Dynamics of biological macromolecules: not a simple slaving by hydration water.Biophys J. 2010 Apr 7;98(7):1321-6. doi: 10.1016/j.bpj.2009.12.4284. Biophys J. 2010. PMID: 20371332 Free PMC article.
References
-
- Smith, J. C. 1991. Protein dynamics: comparison of simulations with inelastic neutron scattering experiments. Q. Rev. Biophys. 24:227–291. - PubMed
-
- Gabel, F., D. Bicout, U. Lehnert, M. Tehei, M. Weik, and G. Zaccai. 2002. Protein dynamics studied by neutron scattering. Q. Rev. Biophys. 35:327–367. - PubMed
-
- Ruffle, S. V., I. Michalarias, J. C. Li, and R. C. Ford. 2002. Inelastic incoherent neutron scattering studies of water interacting with biological macromolecules. J. Am. Chem. Soc. 124:565–569. - PubMed
-
- Bellissent-Funel, M. C. 2003. Status of experiments probing the dynamics of water in confinement. Eur. Phys. J. E Soft Matter. 12:83–92. - PubMed
-
- Doster, W., and M. Settles. 2005. Protein-water displacement distributions. Biochim. Biophys. Acta. 1749:173–186. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

