La Crosse bunyavirus nonstructural protein NSs serves to suppress the type I interferon system of mammalian hosts
- PMID: 17344298
- PMCID: PMC1900204
- DOI: 10.1128/JVI.01933-06
La Crosse bunyavirus nonstructural protein NSs serves to suppress the type I interferon system of mammalian hosts
Abstract
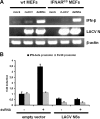
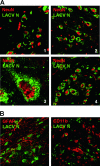
La Crosse virus (LACV) is a mosquito-transmitted member of the Bunyaviridae family that causes severe encephalitis in children. For the LACV nonstructural protein NSs, previous overexpression studies with mammalian cells had suggested two different functions, namely induction of apoptosis and inhibition of RNA interference (RNAi). Here, we demonstrate that mosquito cells persistently infected with LACV do not undergo apoptosis and mount a specific RNAi response. Recombinant viruses that either express (rLACV) or lack (rLACVdelNSs) the NSs gene similarly persisted and were prone to the RNAi-mediated resistance to superinfection. Furthermore, in mosquito cells overexpressed LACV NSs was unable to inhibit RNAi against Semliki Forest virus. In mammalian cells, however, the rLACVdelNSs mutant virus strongly activated the antiviral type I interferon (IFN) system, whereas rLACV as well as overexpressed NSs suppressed IFN induction. Consequently, rLACVdelNSs was attenuated in IFN-competent mouse embryo fibroblasts and animals but not in systems lacking the type I IFN receptor. In situ analyses of mouse brains demonstrated that wild-type and mutant LACV mainly infect neuronal cells and that NSs is able to suppress IFN induction in the central nervous system. Thus, our data suggest little relevance of the NSs-induced apoptosis or RNAi inhibition for growth or pathogenesis of LACV in the mammalian host and indicate that NSs has no function in the insect vector. Since deletion of the viral NSs gene can be fully complemented by inactivation of the host's IFN system, we propose that the major biological function of NSs is suppression of the mammalian innate immune response.
Figures

Similar articles
-
Interferon antagonist NSs of La Crosse virus triggers a DNA damage response-like degradation of transcribing RNA polymerase II.J Biol Chem. 2011 Feb 4;286(5):3681-92. doi: 10.1074/jbc.M110.154799. Epub 2010 Nov 30. J Biol Chem. 2011. PMID: 21118815 Free PMC article.
-
La Crosse virus nonstructural protein NSs counteracts the effects of short interfering RNA.J Virol. 2005 Jan;79(1):234-44. doi: 10.1128/JVI.79.1.234-244.2005. J Virol. 2005. PMID: 15596819 Free PMC article.
-
Efficient cDNA-based rescue of La Crosse bunyaviruses expressing or lacking the nonstructural protein NSs.J Virol. 2005 Aug;79(16):10420-8. doi: 10.1128/JVI.79.16.10420-10428.2005. J Virol. 2005. PMID: 16051834 Free PMC article.
-
Innate immune response to La Crosse virus infection.J Neurovirol. 2014 Apr;20(2):150-6. doi: 10.1007/s13365-013-0186-6. Epub 2013 Jul 12. J Neurovirol. 2014. PMID: 23846288 Review.
-
Phleboviruses and the Type I Interferon Response.Viruses. 2016 Jun 22;8(6):174. doi: 10.3390/v8060174. Viruses. 2016. PMID: 27338447 Free PMC article. Review.
Cited by
-
Visualizing production of beta interferon by astrocytes and microglia in brain of La Crosse virus-infected mice.J Virol. 2012 Oct;86(20):11223-30. doi: 10.1128/JVI.01093-12. Epub 2012 Aug 8. J Virol. 2012. PMID: 22875966 Free PMC article.
-
Genetic characterization of the Wyeomyia group of orthobunyaviruses and their phylogenetic relationships.J Gen Virol. 2012 May;93(Pt 5):1023-1034. doi: 10.1099/vir.0.039479-0. Epub 2012 Jan 25. J Gen Virol. 2012. PMID: 22278828 Free PMC article.
-
Incoming RNA virus nucleocapsids containing a 5'-triphosphorylated genome activate RIG-I and antiviral signaling.Cell Host Microbe. 2013 Mar 13;13(3):336-46. doi: 10.1016/j.chom.2013.01.012. Cell Host Microbe. 2013. PMID: 23498958 Free PMC article.
-
Throw out the Map: Neuropathogenesis of the Globally Expanding California Serogroup of Orthobunyaviruses.Viruses. 2019 Aug 29;11(9):794. doi: 10.3390/v11090794. Viruses. 2019. PMID: 31470541 Free PMC article. Review.
-
Molecular biology of rift valley Fever virus.Open Virol J. 2010 Apr 22;4:8-14. doi: 10.2174/1874357901004020008. Open Virol J. 2010. PMID: 20517489 Free PMC article.
References
-
- Beaty, B. J., D. H. Bishop, M. Gay, and F. Fuller. 1983. Interference between bunyaviruses in Aedes triseriatus mosquitoes. Virology 127:83-90. - PubMed
-
- Benedict, C. A., P. S. Norris, and C. F. Ware. 2002. To kill or be killed: viral evasion of apoptosis. Nat. Immunol. 3:1013-1018. - PubMed
-
- Blakqori, G., G. Kochs, O. Haller, and F. Weber. 2003. Functional L polymerase of La Crosse virus allows in vivo reconstitution of recombinant nucleocapsids. J. Gen. Virol. 84:1207-1214. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI032543-11/AI/NIAID NIH HHS/United States
- R01 AI032543-13/AI/NIAID NIH HHS/United States
- R01 AI034014/AI/NIAID NIH HHS/United States
- R01 AI032543/AI/NIAID NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- R01 AI034014-11/AI/NIAID NIH HHS/United States
- R01 AI034014-12/AI/NIAID NIH HHS/United States
- R01 AI034014-13/AI/NIAID NIH HHS/United States
- R01 AI032543-10/AI/NIAID NIH HHS/United States
- AI32543/AI/NIAID NIH HHS/United States
- AI34014/AI/NIAID NIH HHS/United States
- R01 AI032543-09/AI/NIAID NIH HHS/United States
- R01 AI032543-12/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

