Neuropilins and their ligands are important in the migration of gonadotropin-releasing hormone neurons
- PMID: 17329436
- PMCID: PMC6673474
- DOI: 10.1523/JNEUROSCI.5075-06.2007
Neuropilins and their ligands are important in the migration of gonadotropin-releasing hormone neurons
Abstract
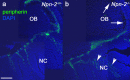
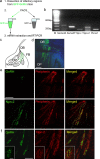
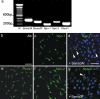
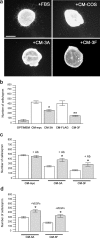
Gonadotropin-releasing hormone (GnRH) neurons in the hypothalamus play an important role in reproductive function. These cells originate in the nasal compartment and migrate into the basal forebrain in association with olfactory/vomeronasal nerves in embryonic life in rodents. Here, we studied the role of neuropilins and their ligands, semaphorins, in the development of the olfactory-GnRH system. We focused on Neuropilin-2 knock-out (Npn-2(-/-)) mice, because they are known to display defasciculation of olfactory nerves and reduced fertility. We found a significant decrease in the number of GnRH neurons in the hypothalamus and a marked reduction in their gonadal size. We then observed an abnormal increase of GnRH neurons in the noses of Npn-2(-/-) mice, indicating that these cells failed to migrate into the forebrain. However, because neuropilins and semaphorins are involved in events of neuronal migration in the brain, we asked whether the observed reduction in GnRH neurons was directly attributable to the action of these molecules. Using fluorescence-activated cell sorting and reverse transcription-PCR on mRNA derived from embryonic green fluorescent protein (GFP)-GnRH transgenic mice, we found expression of class 3 semaphorins and their receptors (neuropilin-1/2 and plexin-A1) in GnRH neurons. Furthermore, double-immunofluorescence experiments showed that migrating GnRH neurons, as well as associated olfactory fibers, express Npn-2 in the nasal region. We then used a line of immortalized GnRH neurons (GN11 cells) that display the same expression patterns for semaphorins and their receptors as GFP-GnRH cells and found that class 3 semaphorins and vascular endothelial growth factors modulate their migratory activity. These studies provide support for the direct involvement of neuropilins and their ligands in the establishment of the GnRH neuroendocrine system.
Figures

Similar articles
-
Semaphorin Signaling in GnRH Neurons: From Development to Disease.Neuroendocrinology. 2019;109(3):193-199. doi: 10.1159/000495916. Epub 2018 Dec 2. Neuroendocrinology. 2019. PMID: 30504719 Review.
-
Dysregulation of Semaphorin7A/β1-integrin signaling leads to defective GnRH-1 cell migration, abnormal gonadal development and altered fertility.Hum Mol Genet. 2011 Dec 15;20(24):4759-74. doi: 10.1093/hmg/ddr403. Epub 2011 Sep 8. Hum Mol Genet. 2011. PMID: 21903667 Free PMC article.
-
Hepatocyte growth factor acts as a motogen and guidance signal for gonadotropin hormone-releasing hormone-1 neuronal migration.J Neurosci. 2007 Jan 10;27(2):431-45. doi: 10.1523/JNEUROSCI.4979-06.2007. J Neurosci. 2007. PMID: 17215404 Free PMC article.
-
Factors involved in the migration of neuroendocrine hypothalamic neurons.Arch Ital Biol. 2005 Sep;143(3-4):171-8. Arch Ital Biol. 2005. PMID: 16097493 Review.
-
Stromal cell-derived factor-1 (chemokine C-X-C motif ligand 12) and chemokine C-X-C motif receptor 4 are required for migration of gonadotropin-releasing hormone neurons to the forebrain.J Neurosci. 2006 Jun 21;26(25):6834-40. doi: 10.1523/JNEUROSCI.1728-06.2006. J Neurosci. 2006. PMID: 16793890 Free PMC article.
Cited by
-
Tumor Necrosis Factor-α Impairs Kisspeptin Signaling in Human Gonadotropin-Releasing Hormone Primary Neurons.J Clin Endocrinol Metab. 2017 Jan 1;102(1):46-56. doi: 10.1210/jc.2016-2115. J Clin Endocrinol Metab. 2017. PMID: 27736314 Free PMC article.
-
CXC chemokine receptor 7 (CXCR7) affects the migration of GnRH neurons by regulating CXCL12 availability.J Neurosci. 2013 Oct 30;33(44):17527-37. doi: 10.1523/JNEUROSCI.0857-13.2013. J Neurosci. 2013. PMID: 24174685 Free PMC article.
-
Puberty, A Sensitive Window of Hypothalamic Development and Plasticity.Endocrinology. 2021 Jan 1;162(1):bqaa209. doi: 10.1210/endocr/bqaa209. Endocrinology. 2021. PMID: 33175140 Free PMC article. Review.
-
Altered proliferative ability of neuronal progenitors in PlexinA1 mutant mice.J Comp Neurol. 2016 Feb 15;524(3):518-34. doi: 10.1002/cne.23806. Epub 2015 Jul 1. J Comp Neurol. 2016. PMID: 25975775 Free PMC article.
-
Early B-cell factors 2 and 3 (EBF2/3) regulate early migration of Cajal-Retzius cells from the cortical hem.Dev Biol. 2012 May 1;365(1):277-89. doi: 10.1016/j.ydbio.2012.02.034. Epub 2012 Mar 6. Dev Biol. 2012. PMID: 22421355 Free PMC article.
References
-
- Bagnard D, Vaillant C, Khuth ST, Dufay N, Lohrum M, Puschel AW, Belin MF, Bolz J, Thomasset N. Semaphorin 3A-vascular endothelial growth factor-165 balance mediates migration and apoptosis of neural progenitor cells by the recruitment of shared receptor. J Neurosci. 2001;21:3332–3341. - PMC - PubMed
-
- Barry J, Hoffman GE, Wray S. LHRH-containing systems. In: Björklund A, Hökfelt T, editors. Handbook of chemical neuroanatomy. Pt I. Vol 4. Amsterdam: Elsevier; 1985. pp. 166–215.
-
- Cariboni A, Pimpinelli F, Colamarino S, Zaninetti R, Piccolella M, Rumio C, Piva F, Rugarli EI, Maggi R. The product of X-linked Kallmann's syndrome gene (KAL1) affects the migratory activity of gonadotropin-releasing hormone (GnRH)-producing neurons. Hum Mol Genet. 2004;13:2781–2791. - PubMed
-
- Cariboni A, Rakic S, Liapi A, Maggi R, Goffinet A, Parnavelas JG. Reelin provides an inhibitory signal in the migration of gonadotropin-releasing hormone neurons. Development. 2005;132:4709–4718. - PubMed
-
- Castellani V, Falk J, Rougon G. Semaphorin3A-induced receptor endocytosis during axon guidance responses is mediated by L1 CAM. Mol Cell Neurosci. 2004;26:89–100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

