Effect of acute exercise on AMPK signaling in skeletal muscle of subjects with type 2 diabetes: a time-course and dose-response study
- PMID: 17327455
- PMCID: PMC2844111
- DOI: 10.2337/db06-1119
Effect of acute exercise on AMPK signaling in skeletal muscle of subjects with type 2 diabetes: a time-course and dose-response study
Abstract
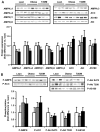
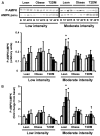
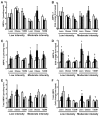
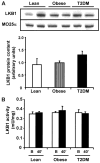
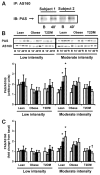
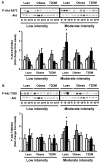
Activation of AMP-activated protein kinase (AMPK) by exercise induces several cellular processes in muscle. Exercise activation of AMPK is unaffected in lean (BMI approximately 25 kg/m(2)) subjects with type 2 diabetes. However, most type 2 diabetic subjects are obese (BMI >30 kg/m(2)), and exercise stimulation of AMPK is blunted in obese rodents. We examined whether obese type 2 diabetic subjects have impaired exercise stimulation of AMPK, at different signaling levels, spanning from the upstream kinase, LKB1, to the putative AMPK targets, AS160 and peroxisome proliferator-activated receptor coactivator (PGC)-1alpha, involved in glucose transport regulation and mitochondrial biogenesis, respectively. Twelve type 2 diabetic, eight obese, and eight lean subjects exercised on a cycle ergometer for 40 min. Muscle biopsies were done before, during, and after exercise. Subjects underwent this protocol on two occasions, at low (50% Vo(2max)) and moderate (70% Vo(2max)) intensities, with a 4-6 week interval. Exercise had no effect on LKB1 activity. Exercise had a time- and intensity-dependent effect to increase AMPK activity and AS160 phosphorylation. Obese and type 2 diabetic subjects had attenuated exercise-stimulated AMPK activity and AS160 phosphorylation. Type 2 diabetic subjects had reduced basal PGC-1 gene expression but normal exercise-induced increases in PGC-1 expression. Our findings suggest that obese type 2 diabetic subjects may need to exercise at higher intensity to stimulate the AMPK-AS160 axis to the same level as lean subjects.
Figures

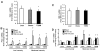
Similar articles
-
LKB1-AMPK signaling in muscle from obese insulin-resistant Zucker rats and effects of training.Am J Physiol Endocrinol Metab. 2006 May;290(5):E925-32. doi: 10.1152/ajpendo.00429.2005. Epub 2005 Dec 13. Am J Physiol Endocrinol Metab. 2006. PMID: 16352671
-
Exercise increases TBC1D1 phosphorylation in human skeletal muscle.Am J Physiol Endocrinol Metab. 2011 Jul;301(1):E164-71. doi: 10.1152/ajpendo.00042.2011. Epub 2011 Apr 19. Am J Physiol Endocrinol Metab. 2011. PMID: 21505148 Free PMC article. Clinical Trial.
-
AMP-activated protein kinase (AMPK) is activated in muscle of subjects with type 2 diabetes during exercise.Diabetes. 2001 May;50(5):921-7. doi: 10.2337/diabetes.50.5.921. Diabetes. 2001. PMID: 11334434
-
Insulin resistance and improvements in signal transduction.Endocrine. 2006 Feb;29(1):73-80. doi: 10.1385/ENDO:29:1:73. Endocrine. 2006. PMID: 16622294 Review.
-
Signaling mechanisms in skeletal muscle: acute responses and chronic adaptations to exercise.IUBMB Life. 2008 Mar;60(3):145-53. doi: 10.1002/iub.21. IUBMB Life. 2008. PMID: 18380005 Free PMC article. Review.
Cited by
-
Regulation of PGC-1α Isoform Expression in Skeletal Muscles.Acta Naturae. 2015 Jan-Mar;7(1):48-59. Acta Naturae. 2015. PMID: 25927001 Free PMC article.
-
Omega-3 fatty acid supplementation combined with acute aerobic exercise does not alter the improved post-exercise insulin response in normoglycemic, inactive and overweight men.Eur J Appl Physiol. 2016 Jun;116(6):1255-65. doi: 10.1007/s00421-016-3387-x. Epub 2016 May 7. Eur J Appl Physiol. 2016. PMID: 27155848 Clinical Trial.
-
Population-scale skeletal muscle single-nucleus multi-omic profiling reveals extensive context specific genetic regulation.bioRxiv [Preprint]. 2024 Dec 17:2023.12.15.571696. doi: 10.1101/2023.12.15.571696. bioRxiv. 2024. PMID: 38168419 Free PMC article. Preprint.
-
The effects of aging, physical training, and a single bout of exercise on mitochondrial protein expression in human skeletal muscle.Exp Gerontol. 2012 Jun;47(6):417-24. doi: 10.1016/j.exger.2012.03.004. Epub 2012 Mar 17. Exp Gerontol. 2012. PMID: 22449457 Free PMC article.
-
Changes in metabolism but not myocellular signaling by training with CHO-restriction in endurance athletes.Physiol Rep. 2018 Sep;6(17):e13847. doi: 10.14814/phy2.13847. Physiol Rep. 2018. PMID: 30175557 Free PMC article. Clinical Trial.
References
-
- Winder WW, Hardie DG. Inactivation of acetyl-CoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am J Physiol. 1996;270:E299–E304. - PubMed
-
- Vavvas D, Apazidis A, Saha AK, Gamble J, Patel A, Kemp BE, Witters LA, Ruderman NB. Contraction-induced changes in acetyl-CoA carboxylase and 5′-AMP-activated kinase in skeletal muscle. J Biol Chem. 1997;272:13255–13261. - PubMed
-
- Hayashi T, Hirshman MF, Kurth EJ, Winder WW, Goodyear LJ. Evidence for 5′ AMP-activated protein kinase mediation of the effect of muscle contraction on glucose transport. Diabetes. 1998;47:1369–1373. - PubMed
-
- Mu J, Brozinick JT, Jr, Valladares O, Bucan M, Birnbaum MJ. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol Cell. 2001;7:1085–1094. - PubMed
-
- Musi N, Hayashi T, Fujii N, Hirshman MF, Witters LA, Goodyear LJ. AMP-activated protein kinase activity and glucose uptake in rat skeletal muscle. Am J Physiol Endocrinol Metab. 2001;280:E677–E684. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK 24092/DK/NIDDK NIH HHS/United States
- R01 DK024092/DK/NIDDK NIH HHS/United States
- MC_U127088492/MRC_/Medical Research Council/United Kingdom
- R01 DK024092-27/DK/NIDDK NIH HHS/United States
- F32 HL086089-03/HL/NHLBI NIH HHS/United States
- R01 DK067690/DK/NIDDK NIH HHS/United States
- L60 MD000986-03A2/MD/NIMHD NIH HHS/United States
- DK 067690/DK/NIDDK NIH HHS/United States
- R01 DK079195/DK/NIDDK NIH HHS/United States
- R56 DK024092/DK/NIDDK NIH HHS/United States
- L60 MD000986/MD/NIMHD NIH HHS/United States
- R01 DK067690-05/DK/NIDDK NIH HHS/United States
- F32 HL086089/HL/NHLBI NIH HHS/United States

