CD4 cells can be more efficient at tumor rejection than CD8 cells
- PMID: 17327412
- PMCID: PMC1890845
- DOI: 10.1182/blood-2006-10-051318
CD4 cells can be more efficient at tumor rejection than CD8 cells
Abstract
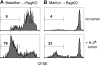
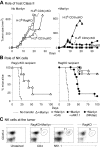
Researchers designing antitumor treatments have long focused on eliciting tumor-specific CD8 cytotoxic T lymphocytes (CTL) because of their potent killing activity and their ability to reject transplanted organs. The resulting treatments, however, have generally been surprisingly poor at inducing complete tumor rejection, both in experimental models and in the clinic. Although a few scattered studies suggested that CD4 T "helper" cells might also serve as antitumor effectors, they have generally been studied mostly for their ability to enhance the activity of CTL. In this mouse study, we compared monoclonal populations of tumor-specific CD4 and CD8 T cells as effectors against several different tumors, and found that CD4 T cells eliminated tumors that were resistant to CD8-mediated rejection, even in cases where the tumors expressed major histocompatibility complex (MHC) class I molecules but not MHC class II. MHC class II expression on host tissues was critical, suggesting that the CD4 T cells act indirectly. Indeed, the CD4 T cells partnered with NK cells to obtain the maximal antitumor effect. These findings suggest that CD4 T cells can be powerful antitumor effector cells that can, in some cases, outperform CD8 T cells, which are the current "gold standard" effector cell in tumor immunotherapy.
Figures

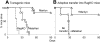

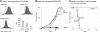

Similar articles
-
Divergent roles for CD4+ T cells in the priming and effector/memory phases of adoptive immunotherapy.J Immunol. 2000 Oct 15;165(8):4246-53. doi: 10.4049/jimmunol.165.8.4246. J Immunol. 2000. PMID: 11035058
-
Tumour-specific CTL response requiring interactions of four different cell types and recognition of MHC class I and class II restricted tumour antigens.Immunol Cell Biol. 1993 Aug;71 ( Pt 4):311-26. doi: 10.1038/icb.1993.36. Immunol Cell Biol. 1993. PMID: 7901150
-
T-cell receptor transgenic analysis of tumor-specific CD8 and CD4 responses in the eradication of solid tumors.Cancer Res. 1999 Mar 1;59(5):1071-9. Cancer Res. 1999. PMID: 10070965
-
Genetic approaches for the induction of a CD4+ T cell response in cancer immunotherapy.J Gene Med. 2005 Jun;7(6):686-95. doi: 10.1002/jgm.713. J Gene Med. 2005. PMID: 15693037 Review.
-
[Recent research advances on function of CD4+T lymphocytes].Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2010 Apr;18(2):544-8. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2010. PMID: 20416207 Review. Chinese.
Cited by
-
Mass Cytometry Reveals Classical Monocytes, NK Cells, and ICOS+ CD4+ T Cells Associated with Pembrolizumab Efficacy in Patients with Lung Cancer.Clin Cancer Res. 2022 Dec 1;28(23):5136-5148. doi: 10.1158/1078-0432.CCR-22-1386. Clin Cancer Res. 2022. PMID: 36166003 Free PMC article.
-
Immunoglobulin Fc fragment tagging allows strong activation of endogenous CD4 T cells to reshape the tumor milieu and enhance the antitumor effect of lentivector immunization.J Immunol. 2012 May 15;188(10):4819-27. doi: 10.4049/jimmunol.1103512. Epub 2012 Apr 13. J Immunol. 2012. PMID: 22504640 Free PMC article.
-
IL-7 signaling imparts polyfunctionality and stemness potential to CD4(+) T cells.Oncoimmunology. 2016 Apr 25;5(6):e1171445. doi: 10.1080/2162402X.2016.1171445. eCollection 2016 Jun. Oncoimmunology. 2016. PMID: 27471650 Free PMC article.
-
Cancer-targeted IL-12 controls human rhabdomyosarcoma by senescence induction and myogenic differentiation.Oncoimmunology. 2015 Mar 19;4(7):e1014760. doi: 10.1080/2162402X.2015.1014760. eCollection 2015 Jul. Oncoimmunology. 2015. PMID: 26140238 Free PMC article.
-
Concomitant targeting of tumor cells and induction of T-cell response synergizes to effectively inhibit trastuzumab-resistant breast cancer.Cancer Res. 2012 Sep 1;72(17):4417-28. doi: 10.1158/0008-5472.CAN-12-1339-T. Epub 2012 Jul 6. Cancer Res. 2012. PMID: 22773664 Free PMC article.
References
-
- Lee KH, Wang E, Nielsen MB, et al. Increased vaccine-specific T cell frequency after peptide-based vaccination correlates with increased susceptibility to in vitro stimulation but does not lead to tumor regression. J Immunol. 1999;163:6292–6300. - PubMed
-
- Peterson AC, Harlin H, Gajewski TF. Immunization with Melan-A peptide-pulsed peripheral blood mononuclear cells plus recombinant human interleukin-12 induces clinical activity and T-cell responses in advanced melanoma. J Clin Oncol. 2003;21:2342–2348. - PubMed
-
- Boon T, Coulie PG, Eynde BJ, Bruggen PV. Human T cell responses against melanoma. Annu Rev Immunol. 2006;24:175–208. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

