PACSIN3 overexpression increases adipocyte glucose transport through GLUT1
- PMID: 17320047
- PMCID: PMC1855247
- DOI: 10.1016/j.bbrc.2007.02.025
PACSIN3 overexpression increases adipocyte glucose transport through GLUT1
Abstract
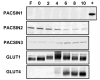
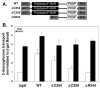
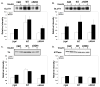
PACSIN family members regulate intracellular vesicle trafficking via their ability to regulate cytoskeletal rearrangement. These processes are known to be involved in trafficking of GLUT1 and GLUT4 in adipocytes. In this study, PACSIN3 was observed to be the only PACSIN isoform that increases in expression during 3T3-L1 adipocyte differentiation. Overexpression of PACSIN3 in 3T3-L1 adipocytes caused an elevation of glucose uptake. Subcellular fractionation revealed that PACSIN3 overexpression elevated GLUT1 plasma membrane localization without effecting GLUT4 distribution. In agreement with this result, examination of GLUT exofacial presentation at the cell surface by photoaffinity labeling revealed significantly increased GLUT1, but not GLUT4, after overexpression of PACSIN3. These results establish a role for PACSIN3 in regulating glucose uptake in adipocytes via its preferential participation in GLUT1 trafficking. They are consistent with the proposal, which is supported by a recent study, that GLUT1, but not GLUT4, is predominantly endocytosed via the coated pit pathway in unstimulated 3T3-L1 adipocytes.
Figures
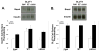
Similar articles
-
Green tea (-)-epigallocatechin gallate suppresses IGF-I and IGF-II stimulation of 3T3-L1 adipocyte glucose uptake via the glucose transporter 4, but not glucose transporter 1 pathway.Gen Comp Endocrinol. 2014 Apr 1;199:46-55. doi: 10.1016/j.ygcen.2014.01.008. Epub 2014 Jan 28. Gen Comp Endocrinol. 2014. PMID: 24486085
-
Beta3-adrenergic receptors stimulate glucose uptake in brown adipocytes by two mechanisms independently of glucose transporter 4 translocation.Endocrinology. 2006 Dec;147(12):5730-9. doi: 10.1210/en.2006-0242. Epub 2006 Sep 7. Endocrinology. 2006. PMID: 16959848
-
Activation of the mammalian target of rapamycin pathway acutely inhibits insulin signaling to Akt and glucose transport in 3T3-L1 and human adipocytes.Endocrinology. 2005 Mar;146(3):1328-37. doi: 10.1210/en.2004-0777. Epub 2004 Dec 2. Endocrinology. 2005. PMID: 15576463
-
Glucose deprivation induces Akt-dependent synthesis and incorporation of GLUT1, but not of GLUT4, into the plasma membrane of 3T3-L1 adipocytes.Eur J Cell Biol. 2000 Dec;79(12):943-9. doi: 10.1078/0171-9335-00118. Eur J Cell Biol. 2000. PMID: 11152285
-
Glut 1 in Cancer Cells and the Inhibitory Action of Resveratrol as A Potential Therapeutic Strategy.Int J Mol Sci. 2019 Jul 9;20(13):3374. doi: 10.3390/ijms20133374. Int J Mol Sci. 2019. PMID: 31324056 Free PMC article. Review.
Cited by
-
Syndapin/SDPN-1 is required for endocytic recycling and endosomal actin association in the C. elegans intestine.Mol Biol Cell. 2016 Sep 14;27(23):3746-56. doi: 10.1091/mbc.E16-02-0116. Online ahead of print. Mol Biol Cell. 2016. PMID: 27630264 Free PMC article.
-
F-BAR family proteins, emerging regulators for cell membrane dynamic changes-from structure to human diseases.J Hematol Oncol. 2015 May 9;8:47. doi: 10.1186/s13045-015-0144-2. J Hematol Oncol. 2015. PMID: 25956236 Free PMC article. Review.
-
Variant-to-gene-mapping analyses reveal a role for pancreatic islet cells in conferring genetic susceptibility to sleep-related traits.Sleep. 2022 Aug 11;45(8):zsac109. doi: 10.1093/sleep/zsac109. Sleep. 2022. PMID: 35537191 Free PMC article.
-
PACSIN proteins in vivo: Roles in development and physiology.Acta Physiol (Oxf). 2022 Mar;234(3):e13783. doi: 10.1111/apha.13783. Epub 2022 Jan 20. Acta Physiol (Oxf). 2022. PMID: 34990060 Free PMC article. Review.
-
Cloning, purification, crystallization and preliminary X-ray diffraction analysis of mouse PACSIN 3 protein.Acta Crystallogr Sect F Struct Biol Cryst Commun. 2012 Feb 1;68(Pt 2):159-62. doi: 10.1107/S1744309111049116. Epub 2012 Jan 25. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2012. PMID: 22297988 Free PMC article.
References
-
- Joost H, Bell GI, Best JD, Birnbaum MJ, Charron MJ, Chen YT, Doege H, James DE, Lodish HF, Moley KH, Moley JF, Mueckler M, Rogers S, Shurmann A, Seino S, Thorens B. Nomenclature of the GLUT/SLC2A family of sugar/polyol transport facilitators. Am J Physiol Endocrinol Metab. 2001;282:E974–E976. - PubMed
-
- Yang JH, Holman GD. Comparison of GLUT4 and GLUT1 subcellular trafficking in basal and insulin-stimulated 3T3-L1 cells. J Biol Chem. 1993;268:4600–4603. - PubMed
-
- Watson RT, Kanzaki M, Pessin JE. Regulated membrane trafficking of the insulin-responsive glucose transporter 4 in adipocytes. Endocr Rev. 2004;25:177–204. - PubMed
-
- Wasiak S, Quinn CC, Ritter B, de Heuvel E, Baranes D, Plomann M, McPherson PS. The Ras/Rac guanine nucleotide exchange factor mammalian Son-of-sevenless interacts with PACSIN 1/Syndapin I, a regulator of endocytosis and the actin cytoskeleton. J Biol Chem. 2001;276:26622–26628. - PubMed
-
- da Costa SR, Sou E, Xie J, Yarber FA, Okamoto CT, Pdigeon M, Kessels MM, Mircheff AK, Schechter JE, Qualmann B, Hamm-Alvarez SF. Impairing actin filament or syndapin functions promotes accumulation of clathrin-coated vesicles at the apical plasma membrane of acinar epithelial cells. Mol Biol Cell. 2003;14:4397–4413. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

