ERK activity and G1 phase progression: identifying dispensable versus essential activities and primary versus secondary targets
- PMID: 17314399
- PMCID: PMC1838994
- DOI: 10.1091/mbc.e06-10-0908
ERK activity and G1 phase progression: identifying dispensable versus essential activities and primary versus secondary targets
Abstract
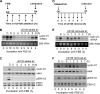
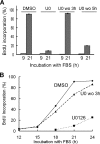
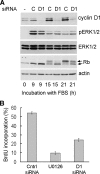
The ERK subfamily of MAP kinases is a critical regulator of S phase entry. ERK activity regulates the induction of cyclin D1, and a sustained ERK signal is thought to be required for this effect, at least in fibroblasts. We now show that early G1 phase ERK activity is dispensable for the induction of cyclin D1 and that the critical ERK signaling period is restricted to 3-6 h after mitogenic stimulation of quiescent fibroblasts. Similarly, early G1 phase ERK activity is dispensable for entry into S phase. Moreover, if cyclin D1 is expressed ectopically, ERK activity becomes dispensable throughout the G1 phase. In addition to its effect on cyclin D1, ERK activity is thought to contribute to the down-regulation of p27kip1. We found that this effect is restricted to late G1/S phase. Mechanistic analysis showed that the ERK effect on p27kip1 is mediated by Skp2 and is secondary to its effect on cyclin D1. Our results emphasize the importance of mid-G1 phase ERK activity and resolve primary versus secondary ERK targets within the G1 phase cyclin-dependent kinases.
Figures


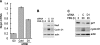
Similar articles
-
Joint requirement for Rac and ERK activities underlies the mid-G1 phase induction of cyclin D1 and S phase entry in both epithelial and mesenchymal cells.J Biol Chem. 2008 Nov 7;283(45):30911-8. doi: 10.1074/jbc.M804537200. Epub 2008 Aug 20. J Biol Chem. 2008. PMID: 18715870 Free PMC article.
-
Adhesion control of cyclin D1 and p27Kip1 levels is deregulated in melanoma cells through BRAF-MEK-ERK signaling.Oncogene. 2005 May 12;24(21):3459-71. doi: 10.1038/sj.onc.1208544. Oncogene. 2005. PMID: 15735667
-
Mitogenic activity of estrogens in human breast cancer cells does not rely on direct induction of mitogen-activated protein kinase/extracellularly regulated kinase or phosphatidylinositol 3-kinase.Mol Endocrinol. 2004 Nov;18(11):2700-13. doi: 10.1210/me.2003-0133. Epub 2004 Aug 5. Mol Endocrinol. 2004. PMID: 15297603
-
Integrin-dependent signal transduction regulating cyclin D1 expression and G1 phase cell cycle progression.Cancer Metastasis Rev. 2005 Sep;24(3):383-93. doi: 10.1007/s10555-005-5130-7. Cancer Metastasis Rev. 2005. PMID: 16258726 Review.
-
Regulation of cell cycle entry and G1 progression by CSF-1.Mol Reprod Dev. 1997 Jan;46(1):11-8. doi: 10.1002/(SICI)1098-2795(199701)46:1<11::AID-MRD3>3.0.CO;2-U. Mol Reprod Dev. 1997. PMID: 8981358 Review.
Cited by
-
A CDK4/6 inhibitor enhances cytotoxicity of paclitaxel in lung adenocarcinoma cells harboring mutant KRAS as well as wild-type KRAS.Cancer Biol Ther. 2013 Jul;14(7):597-605. doi: 10.4161/cbt.24592. Epub 2013 May 10. Cancer Biol Ther. 2013. PMID: 23792647 Free PMC article.
-
Transcriptional regulation of the cyclin D1 gene at a glance.J Cell Sci. 2008 Dec 1;121(Pt 23):3853-7. doi: 10.1242/jcs.039131. J Cell Sci. 2008. PMID: 19020303 Free PMC article. Review. No abstract available.
-
Joint requirement for Rac and ERK activities underlies the mid-G1 phase induction of cyclin D1 and S phase entry in both epithelial and mesenchymal cells.J Biol Chem. 2008 Nov 7;283(45):30911-8. doi: 10.1074/jbc.M804537200. Epub 2008 Aug 20. J Biol Chem. 2008. PMID: 18715870 Free PMC article.
-
Wnt-induced, TRP53-mediated Cell Cycle Arrest of Precursors Underlies Interstitial Cell of Cajal Depletion During Aging.Cell Mol Gastroenterol Hepatol. 2021;11(1):117-145. doi: 10.1016/j.jcmgh.2020.07.011. Epub 2020 Aug 7. Cell Mol Gastroenterol Hepatol. 2021. PMID: 32771388 Free PMC article.
-
Proteasome activity is required for the initiation of precancerous pancreatic lesions.Sci Rep. 2016 May 31;6:27044. doi: 10.1038/srep27044. Sci Rep. 2016. PMID: 27244456 Free PMC article.
References
-
- Albanese C., Johnson J., Watanabe G., Eklund N., Vu D., Arnold A., Pestell R. G. Transforming p21ras mutants and c-Ets-2 activate the cyclin D1 promoter through distinguishable regions. J. Biol. Chem. 1995;270:23589–23597. - PubMed
-
- Balmanno K., Cook S. J. Sustained MAP kinase activation is required for the expression of cyclin D1, p21Cip1 and a subset of AP-1 proteins in CCL39 cells. Oncogene. 1999;18:3085–3097. - PubMed
-
- Bhatt K. V., Spofford L. S., Aram G., McMullen M., Pumiglia K., Aplin A. E. Adhesion control of cyclin D1 and p27Kip1 levels is deregulated in melanoma cells through BRAF-MEK-ERK signaling. Oncogene. 2005;24:3459–3471. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

