Formins regulate the actin-related protein 2/3 complex-independent polarization of the centrosome to the immunological synapse
- PMID: 17306570
- PMCID: PMC2836258
- DOI: 10.1016/j.immuni.2007.01.008
Formins regulate the actin-related protein 2/3 complex-independent polarization of the centrosome to the immunological synapse
Abstract
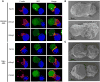
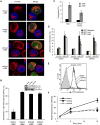
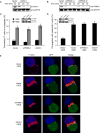
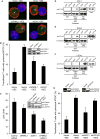
T cell receptor (TCR)-mediated cytoskeletal reorganization is considered to be actin-related protein (Arp) 2/3 complex dependent. We therefore examined the requirement for Arp2/3- and formin-dependent F-actin nucleation during T cell activation. We demonstrated that without Arp2/3-mediated actin nucleation, stimulated T cells could not form an F-actin-rich lamellipod, but instead produced polarized filopodia-like structures. Moreover, the microtubule-organizing center (MTOC, or centrosome), which rapidly reorients to the immunological synapse through an unknown mechanism, polarized in the absence of Arp2/3. Conversely, the actin-nucleating formins, Diaphanous-1 (DIA1) and Formin-like-1 (FMNL1), did not affect TCR-stimulated F-actin-rich structures, but instead displayed unique patterns of centrosome colocalization and controlled TCR-mediated centrosome polarization. Depletion of FMNL1 or DIA1 in cytotoxic lymphocytes abrogated cell-mediated killing. Altogether, our results have identified Arp2/3 complex-independent cytoskeletal reorganization events in T lymphocytes and indicate that formins are essential cytoskeletal regulators of centrosome polarity in T cells.
Conflict of interest statement
Figures



Comment in
-
Formin the way.Immunity. 2007 Feb;26(2):139-41. doi: 10.1016/j.immuni.2007.02.007. Immunity. 2007. PMID: 17307700 Review.
Similar articles
-
Formin the way.Immunity. 2007 Feb;26(2):139-41. doi: 10.1016/j.immuni.2007.02.007. Immunity. 2007. PMID: 17307700 Review.
-
Actin reorganization at the centrosomal area and the immune synapse regulates polarized secretory traffic of multivesicular bodies in T lymphocytes.J Extracell Vesicles. 2020 Jun 19;9(1):1759926. doi: 10.1080/20013078.2020.1759926. J Extracell Vesicles. 2020. PMID: 32939232 Free PMC article.
-
INF2 promotes the formation of detyrosinated microtubules necessary for centrosome reorientation in T cells.J Cell Biol. 2012 Sep 17;198(6):1025-37. doi: 10.1083/jcb.201202137. J Cell Biol. 2012. PMID: 22986496 Free PMC article.
-
Actin nucleation at the centrosome controls lymphocyte polarity.Nat Commun. 2016 Mar 18;7:10969. doi: 10.1038/ncomms10969. Nat Commun. 2016. PMID: 26987298 Free PMC article.
-
Actin cytoskeletal dynamics in T lymphocyte activation and migration.J Leukoc Biol. 2003 Jan;73(1):30-48. doi: 10.1189/jlb.0602272. J Leukoc Biol. 2003. PMID: 12525560 Review.
Cited by
-
Diaphanous-related formin mDia2 regulates beta2 integrins to control hematopoietic stem and progenitor cell engraftment.Nat Commun. 2020 Jun 23;11(1):3172. doi: 10.1038/s41467-020-16911-4. Nat Commun. 2020. PMID: 32576838 Free PMC article.
-
Diacylglycerol promotes centrosome polarization in T cells via reciprocal localization of dynein and myosin II.Proc Natl Acad Sci U S A. 2013 Jul 16;110(29):11976-81. doi: 10.1073/pnas.1306180110. Epub 2013 Jul 1. Proc Natl Acad Sci U S A. 2013. PMID: 23818610 Free PMC article.
-
T cell activation.Annu Rev Immunol. 2009;27:591-619. doi: 10.1146/annurev.immunol.021908.132706. Annu Rev Immunol. 2009. PMID: 19132916 Free PMC article. Review.
-
The formin FMNL3 assembles plasma membrane protrusions that participate in cell-cell adhesion.Mol Biol Cell. 2015 Feb 1;26(3):467-77. doi: 10.1091/mbc.E14-07-1247. Epub 2014 Nov 26. Mol Biol Cell. 2015. PMID: 25428984 Free PMC article.
-
The centrosome is an actin-organizing centre.Nat Cell Biol. 2016 Jan;18(1):65-75. doi: 10.1038/ncb3285. Epub 2015 Dec 14. Nat Cell Biol. 2016. PMID: 26655833 Free PMC article.
References
-
- Ardouin L, Bracke M, Mathiot A, Pagakis SN, Norton T, Hogg N, Tybulewicz VL. Vav1 transduces TCR signals required for LFA-1 function and cell polarization at the immunological synapse. Eur J Immunol. 2003;33:790–797. - PubMed
-
- Barr VA, Balagopalan L, Barda-Saad M, Polishchuk R, Boukari H, Bunnell SC, Bernot KM, Toda Y, Nossal R, Samelson LE. T-cell antigen receptor-induced signaling complexes: internalization via a cholesterol-dependent endocytic pathway. Traffic. 2006;7:1143–1162. - PubMed
-
- Biyasheva A, Svitkina T, Kunda P, Baum B, Borisy G. Cascade pathway of filopodia formation downstream of SCAR. J Cell Sci. 2004;117:837–848. - PubMed
-
- Bunnell SC, Kapoor V, Trible RP, Zhang W, Samelson LE. Dynamic actin polymerization drives T cell receptor-induced spreading: a role for the signal transduction adaptor LAT. Immunity. 2001;14:315–329. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

