Wnt signaling induces matrix metalloproteinase expression and regulates T cell transmigration
- PMID: 17306568
- PMCID: PMC1855210
- DOI: 10.1016/j.immuni.2006.12.007
Wnt signaling induces matrix metalloproteinase expression and regulates T cell transmigration
Abstract
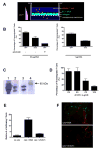
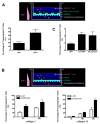
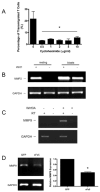
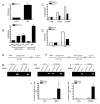
Wnts are a family of secreted glycoproteins with diverse developmental roles, including regulation of cell migration; however, little is known about wnt signaling in mature T cells. We find that endothelial-cell-derived wnts, acting through Frizzled receptors, induce matrix metalloproteinase (MMP) 2 and MMP9 expression in effector T cells. Blocking wnt signaling, or MMP activity, reduces T cell migration through the basement membrane in vitro and into inflamed skin in vivo. Wnt signaling stabilizes beta-catenin protein in T cells and directly targets the MMP promoters through tandem TCF sites. Thus, our data support a necessary and previously unexpected role for wnt signaling in T cell extravasation.
Figures

Similar articles
-
Stably overexpressed human Frizzled-2 signals through the beta-catenin pathway and does not activate Ca2+-mobilization in Human Embryonic Kidney 293 cells.Cell Signal. 2009 Jan;21(1):22-33. doi: 10.1016/j.cellsig.2008.09.008. Epub 2008 Sep 25. Cell Signal. 2009. PMID: 18929644
-
Role of Wnt-5A in interleukin-1beta-induced matrix metalloproteinase expression in rabbit temporomandibular joint condylar chondrocytes.Arthritis Rheum. 2009 Sep;60(9):2714-22. doi: 10.1002/art.24779. Arthritis Rheum. 2009. PMID: 19714632
-
Wnt/beta-catenin signaling induces proliferation, survival and interleukin-8 in human endothelial cells.Angiogenesis. 2005;8(1):43-51. doi: 10.1007/s10456-005-5612-9. Angiogenesis. 2005. PMID: 16132617
-
Multiplicity of the interactions of Wnt proteins and their receptors.Cell Signal. 2007 Apr;19(4):659-71. doi: 10.1016/j.cellsig.2006.11.001. Epub 2006 Nov 16. Cell Signal. 2007. PMID: 17188462 Review.
-
Non-conventional Frizzled ligands and Wnt receptors.Dev Growth Differ. 2008 May;50(4):229-43. doi: 10.1111/j.1440-169X.2008.01016.x. Dev Growth Differ. 2008. PMID: 18366384 Review.
Cited by
-
Human cytomegalovirus infection dysregulates the canonical Wnt/β-catenin signaling pathway.PLoS Pathog. 2012;8(10):e1002959. doi: 10.1371/journal.ppat.1002959. Epub 2012 Oct 11. PLoS Pathog. 2012. PMID: 23071438 Free PMC article.
-
Pharmacological modulation of beta-catenin and its applications in cancer therapy.J Cell Mol Med. 2013 Apr;17(4):449-56. doi: 10.1111/jcmm.12033. Epub 2013 Mar 14. J Cell Mol Med. 2013. PMID: 23490077 Free PMC article. Review.
-
Decorin antagonizes the angiogenic network: concurrent inhibition of Met, hypoxia inducible factor 1α, vascular endothelial growth factor A, and induction of thrombospondin-1 and TIMP3.J Biol Chem. 2012 Feb 17;287(8):5492-506. doi: 10.1074/jbc.M111.283499. Epub 2011 Dec 22. J Biol Chem. 2012. PMID: 22194599 Free PMC article.
-
MSK1-Mediated β-Catenin Phosphorylation Confers Resistance to PI3K/mTOR Inhibitors in Glioblastoma.Mol Cancer Ther. 2016 Jul;15(7):1656-68. doi: 10.1158/1535-7163.MCT-15-0857. Epub 2016 Apr 22. Mol Cancer Ther. 2016. PMID: 27196759 Free PMC article.
-
In vitro modelling of human proprioceptive sensory neurons in the neuromuscular system.Sci Rep. 2022 Dec 9;12(1):21318. doi: 10.1038/s41598-022-23565-3. Sci Rep. 2022. PMID: 36494423 Free PMC article.
References
-
- Alon R, Feigelson S. From rolling to arrest on blood vessels: leukocyte tap dancing on endothelial integrin ligands and chemokines at sub-second contacts. Semin Immunol. 2002;14:93–104. - PubMed
-
- Barker N, Morin PJ, Clevers H. The Yin-Yang of TCF/beta-catenin signaling. Adv Cancer Res. 2000;77:1–24. - PubMed
-
- Cai Y, Chen T, Xu Q. Astilbin suppresses delayed-type hypersensitivity by inhibiting lymphocyte migration. J Pharm Pharmacol. 2003;55:691–696. - PubMed
-
- Cheng CW, Smith SK, Charnock-Jones DS. Wnt-1 signaling inhibits human umbilical vein endothelial cell proliferation and alters cell morphology. Exp Cell Res. 2003;291:415–425. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

