Molecular modeling of the human P2Y2 receptor and design of a selective agonist, 2'-amino-2'-deoxy-2-thiouridine 5'-triphosphate
- PMID: 17302398
- PMCID: PMC3404812
- DOI: 10.1021/jm060903o
Molecular modeling of the human P2Y2 receptor and design of a selective agonist, 2'-amino-2'-deoxy-2-thiouridine 5'-triphosphate
Abstract
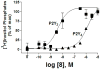
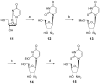
A rhodopsin-based homology model of the nucleotide-activated human P2Y2 receptor, including loops, termini, and phospholipids, was optimized with the Monte Carlo multiple minimum conformational search routine. Docked uridine 5'-triphosphate (UTP) formed a nucleobase pi-pi complex with conserved Phe3.32. Selectivity-enhancing 2'-amino-2'-deoxy substitution interacted through pi-hydrogen-bonding with aromatic Phe6.51 and Tyr3.33. A "sequential ligand composition" approach for docking the flexible dinucleotide agonist Up4U demonstrated a shift of conserved cationic Arg3.29 from the UTP gamma position to the delta position of Up4U and Up4 ribose. Synthesized nucleotides were tested as agonists at human P2Y receptors expressed in 1321N1 astrocytoma cells. 2'-Amino and 2-thio modifications were synergized to enhance potency and selectivity; compound 8 (EC50 = 8 nM) was 300-fold P2Y2-selective versus P2Y4. 2'-Amine acetylation reduced potency, and trifluoroacetylation produced intermediate potency. 5-Amino nucleobase substitution did not enhance P2Y2 potency through a predicted hydrophilic interaction possibly because of destabilization of the receptor-favored Northern conformation of ribose. This detailed view of P2Y2 receptor recognition suggests mutations for model validation.
Figures
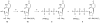
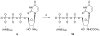
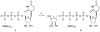
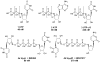
Similar articles
-
Structure activity and molecular modeling analyses of ribose- and base-modified uridine 5'-triphosphate analogues at the human P2Y2 and P2Y4 receptors.Biochem Pharmacol. 2006 Feb 14;71(4):540-9. doi: 10.1016/j.bcp.2005.11.010. Epub 2005 Dec 15. Biochem Pharmacol. 2006. PMID: 16359641 Free PMC article.
-
Synthesis and structure-activity relationships of uracil nucleotide derivatives and analogues as agonists at human P2Y2, P2Y4, and P2Y6 receptors.J Med Chem. 2006 Nov 30;49(24):7076-87. doi: 10.1021/jm060848j. J Med Chem. 2006. PMID: 17125260
-
Methanocarba modification of uracil and adenine nucleotides: high potency of Northern ring conformation at P2Y1, P2Y2, P2Y4, and P2Y11 but not P2Y6 receptors.J Med Chem. 2002 Jan 3;45(1):208-18. doi: 10.1021/jm010369e. J Med Chem. 2002. PMID: 11754592 Free PMC article.
-
Molecular pharmacology of P2Y-receptors.Naunyn Schmiedebergs Arch Pharmacol. 2000 Nov;362(4-5):310-23. doi: 10.1007/s002100000310. Naunyn Schmiedebergs Arch Pharmacol. 2000. PMID: 11111826 Review.
-
Agonists and antagonists for P2 receptors.Novartis Found Symp. 2006;276:58-68; discussion 68-72, 107-12, 275-81. doi: 10.1002/9780470032244.ch6. Novartis Found Symp. 2006. PMID: 16805423 Free PMC article. Review.
Cited by
-
Synthesis and P2Y₂ receptor agonist activities of uridine 5'-phosphonate analogues.Bioorg Med Chem. 2012 Apr 1;20(7):2304-15. doi: 10.1016/j.bmc.2012.02.012. Epub 2012 Feb 15. Bioorg Med Chem. 2012. PMID: 22386981 Free PMC article.
-
UDP is a competitive antagonist at the human P2Y14 receptor.J Pharmacol Exp Ther. 2008 May;325(2):588-94. doi: 10.1124/jpet.108.136309. Epub 2008 Feb 5. J Pharmacol Exp Ther. 2008. PMID: 18252808 Free PMC article.
-
Synthesis and potency of novel uracil nucleotides and derivatives as P2Y2 and P2Y6 receptor agonists.Bioorg Med Chem. 2008 Jun 15;16(12):6319-32. doi: 10.1016/j.bmc.2008.05.013. Epub 2008 May 9. Bioorg Med Chem. 2008. PMID: 18514530 Free PMC article.
-
Molecular Structure of P2Y Receptors: Mutagenesis, Modeling, and Chemical Probes.Wiley Interdiscip Rev Membr Transp Signal. 2012 Sep 12;1(6):WMTS68. doi: 10.1002/wmts.68. Wiley Interdiscip Rev Membr Transp Signal. 2012. PMID: 23336097 Free PMC article.
-
Toward the three-dimensional structure and lysophosphatidic acid binding characteristics of the LPA(4)/p2y(9)/GPR23 receptor: a homology modeling study.J Mol Graph Model. 2009 Aug;28(1):70-9. doi: 10.1016/j.jmgm.2009.04.004. Epub 2009 Apr 19. J Mol Graph Model. 2009. PMID: 19423373 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Molecular Biology Databases
Research Materials
Miscellaneous

