A Drosophila model of mutant human parkin-induced toxicity demonstrates selective loss of dopaminergic neurons and dependence on cellular dopamine
- PMID: 17267552
- PMCID: PMC6673194
- DOI: 10.1523/JNEUROSCI.4810-06.2007
A Drosophila model of mutant human parkin-induced toxicity demonstrates selective loss of dopaminergic neurons and dependence on cellular dopamine
Abstract
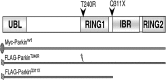
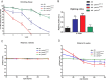
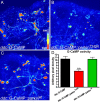
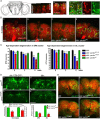
Mutations in human parkin have been identified in familial Parkinson's disease and in some sporadic cases. Here, we report that expression of mutant but not wild-type human parkin in Drosophila causes age-dependent, selective degeneration of dopaminergic (DA) neurons accompanied by a progressive motor impairment. Overexpression or knockdown of the Drosophila vesicular monoamine transporter, which regulates cytosolic DA homeostasis, partially rescues or exacerbates, respectively, the degenerative phenotypes caused by mutant human parkin. These results support a model in which the vulnerability of DA neurons to parkin-induced neurotoxicity results from the interaction of mutant parkin with cytoplasmic dopamine.
Figures
Similar articles
-
Drosophila overexpressing parkin R275W mutant exhibits dopaminergic neuron degeneration and mitochondrial abnormalities.J Neurosci. 2007 Aug 8;27(32):8563-70. doi: 10.1523/JNEUROSCI.0218-07.2007. J Neurosci. 2007. PMID: 17687034 Free PMC article.
-
Parkin suppresses dopaminergic neuron-selective neurotoxicity induced by Pael-R in Drosophila.Neuron. 2003 Mar 27;37(6):911-24. doi: 10.1016/s0896-6273(03)00143-0. Neuron. 2003. PMID: 12670421
-
Bacterial artificial chromosome transgenic mice expressing a truncated mutant parkin exhibit age-dependent hypokinetic motor deficits, dopaminergic neuron degeneration, and accumulation of proteinase K-resistant alpha-synuclein.J Neurosci. 2009 Feb 18;29(7):1962-76. doi: 10.1523/JNEUROSCI.5351-08.2009. J Neurosci. 2009. PMID: 19228951 Free PMC article.
-
What have we learned from Drosophila models of Parkinson's disease?Prog Brain Res. 2010;184:3-16. doi: 10.1016/S0079-6123(10)84001-4. Prog Brain Res. 2010. PMID: 20887867 Review.
-
The synaptic function of parkin.Brain. 2017 Sep 1;140(9):2265-2272. doi: 10.1093/brain/awx006. Brain. 2017. PMID: 28335015 Review.
Cited by
-
Genetic animal models of Parkinson's disease.Neuron. 2010 Jun 10;66(5):646-61. doi: 10.1016/j.neuron.2010.04.034. Neuron. 2010. PMID: 20547124 Free PMC article. Review.
-
Interactions between Tau and α-synuclein augment neurotoxicity in a Drosophila model of Parkinson's disease.Hum Mol Genet. 2014 Jun 1;23(11):3008-23. doi: 10.1093/hmg/ddu011. Epub 2014 Jan 14. Hum Mol Genet. 2014. PMID: 24430504 Free PMC article.
-
Animal models of Parkinson's disease progression.Acta Neuropathol. 2008 Apr;115(4):385-98. doi: 10.1007/s00401-008-0350-x. Epub 2008 Feb 14. Acta Neuropathol. 2008. PMID: 18273623 Free PMC article. Review.
-
Drosophila melanogaster as a model organism of brain diseases.Int J Mol Sci. 2009 Feb;10(2):407-440. doi: 10.3390/ijms10020407. Epub 2009 Feb 2. Int J Mol Sci. 2009. PMID: 19333415 Free PMC article. Review.
-
PINK1-dependent phosphorylation of PINK1 and Parkin is essential for mitochondrial quality control.Cell Death Dis. 2016 Dec 1;7(12):e2501. doi: 10.1038/cddis.2016.396. Cell Death Dis. 2016. PMID: 27906179 Free PMC article.
References
-
- Auluck PK, Bonini NM. Pharmacological prevention of Parkinson disease in Drosophila . Nat Med. 2002;8:1185–1186. - PubMed
-
- Auluck PK, Chan HY, Trojanowski JQ, Lee VM, Bonini NM. Chaperone suppression of alpha-synuclein toxicity in a Drosophila model for Parkinson's disease. Science. 2002;295:865–868. - PubMed
-
- Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, Dekker MC, Squitieri F, Ibanez P, Joosse M, van Dongen JW, Vanacore N, van Swieten JC, Brice A, Meco G, van Duijn CM, Oostra BA, Heutink P. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299:256–259. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
