NFX1-123 and poly(A) binding proteins synergistically augment activation of telomerase in human papillomavirus type 16 E6-expressing cells
- PMID: 17267499
- PMCID: PMC1866132
- DOI: 10.1128/JVI.02007-06
NFX1-123 and poly(A) binding proteins synergistically augment activation of telomerase in human papillomavirus type 16 E6-expressing cells
Abstract
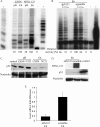
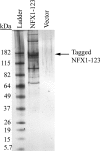
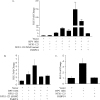
Overcoming senescence signals in somatic cells is critical to cellular immortalization and carcinogenesis. High-risk human papillomavirus (HPV) can immortalize epithelial cells in culture through degradation of the retinoblastoma protein by HPV E7 and activation of hTERT transcription, the catalytic subunit of telomerase, by the heterodimer HPV E6/E6-associated protein (E6AP). Recent work in our laboratory identified a novel repressor of hTERT transcription, NFX1-91, which is targeted for ubiquitin-mediated degradation by HPV type 16 (HPV16) E6/E6AP. In contrast, NFX1-123, a splice variant NFX1, increased expression from an hTERT promoter that was activated by HPV16 E6/E6AP. Here, we show that HPV16 E6 bound both NFX1-91 and NFX1-123 through the common central domain of NFX1 in the absence of E6AP. NFX1-123 positively regulated hTERT expression, as its knockdown decreased hTERT mRNA levels and telomerase activity and its overexpression increased telomerase activity. We identified new protein partners of NFX1-123, including several cytoplasmic poly(A) binding proteins (PABPCs) that interacted with NFX1-123 through its N-terminal PAM2 motif, a protein domain characteristic of other PABPC protein partners. Furthermore, NFX1-123 and PABPCs together had a synergistic stimulatory effect on hTERT-regulated reporter assays. The data suggest that NFX1-123 is integral to hTERT regulation in HPV16 E6-expressing epithelial cells and that the interaction between NFX1-123 and PABPCs is critical to hTERT activity.
Figures

Similar articles
-
Cytoplasmic poly(A) binding proteins regulate telomerase activity and cell growth in human papillomavirus type 16 E6-expressing keratinocytes.J Virol. 2010 Dec;84(24):12934-44. doi: 10.1128/JVI.01377-10. Epub 2010 Oct 13. J Virol. 2010. PMID: 20943973 Free PMC article.
-
NFX1-123 increases hTERT expression and telomerase activity posttranscriptionally in human papillomavirus type 16 E6 keratinocytes.J Virol. 2009 Jul;83(13):6446-56. doi: 10.1128/JVI.02556-08. Epub 2009 Apr 15. J Virol. 2009. PMID: 19369336 Free PMC article.
-
Identification of a novel telomerase repressor that interacts with the human papillomavirus type-16 E6/E6-AP complex.Genes Dev. 2004 Sep 15;18(18):2269-82. doi: 10.1101/gad.1214704. Genes Dev. 2004. PMID: 15371341 Free PMC article.
-
Activation of telomerase by HPVs.Virus Res. 2017 Mar 2;231:50-55. doi: 10.1016/j.virusres.2016.11.003. Epub 2016 Nov 15. Virus Res. 2017. PMID: 27863966 Review.
-
Post-Transcriptional Gene Regulation by HPV 16E6 and Its Host Protein Partners.Viruses. 2022 Jul 6;14(7):1483. doi: 10.3390/v14071483. Viruses. 2022. PMID: 35891463 Free PMC article. Review.
Cited by
-
NFX1 plays a role in human papillomavirus type 16 E6 activation of NFkappaB activity.J Virol. 2010 Nov;84(21):11461-9. doi: 10.1128/JVI.00538-10. Epub 2010 Aug 25. J Virol. 2010. PMID: 20739528 Free PMC article.
-
HPV type 16 E6 and NFX1-123 augment JNK signaling to mediate keratinocyte differentiation and L1 expression.Virology. 2019 May;531:171-182. doi: 10.1016/j.virol.2019.03.008. Epub 2019 Mar 16. Virology. 2019. PMID: 30903928 Free PMC article.
-
Human papillomavirus type 16 E6 and NFX1-123 mislocalize immune signaling proteins and downregulate immune gene expression in keratinocytes.PLoS One. 2017 Nov 8;12(11):e0187514. doi: 10.1371/journal.pone.0187514. eCollection 2017. PLoS One. 2017. PMID: 29117186 Free PMC article.
-
Human papillomavirus 16E6 and NFX1-123 potentiate Notch signaling and differentiation without activating cellular arrest.Virology. 2015 Apr;478:50-60. doi: 10.1016/j.virol.2015.02.002. Epub 2015 Feb 25. Virology. 2015. PMID: 25723053 Free PMC article.
-
Basic mechanisms of high-risk human papillomavirus-induced carcinogenesis: roles of E6 and E7 proteins.Cancer Sci. 2007 Oct;98(10):1505-11. doi: 10.1111/j.1349-7006.2007.00546.x. Epub 2007 Jul 23. Cancer Sci. 2007. PMID: 17645777 Free PMC article. Review.
References
-
- Bartz, S. R., and M. A. Vodicka. 1997. Production of high-titer human immunodeficiency virus type 1 pseudotyped with vesicular stomatitis virus glycoprotein. Methods 12:337-342. - PubMed
-
- Beitzinger, M., C. Oswald, R. Beinoraviciute-Kellner, and T. Stiewe. 2006. Regulation of telomerase activity by the p53 family member p73. Oncogene 25:813-826. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

