Erythropoietin protects cardiac myocytes against anthracycline-induced apoptosis
- PMID: 17250809
- PMCID: PMC1831847
- DOI: 10.1016/j.bbrc.2007.01.044
Erythropoietin protects cardiac myocytes against anthracycline-induced apoptosis
Abstract
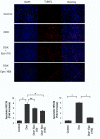
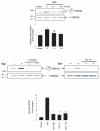
The cardiotoxic adverse effects of anthracycline antibiotics limit their therapeutic utility as essential components of chemotherapy regimens for hematologic and solid malignancies. Here we show that the hematopoietic cytokine erythropoietin attenuates doxorubicin-induced apoptosis of primary neonatal rat ventricular cardiomyocytes in a dose-dependent manner. Erythropoietin treatment induced rapid, time-dependent phosphorylation of MAP kinases (MAPK) Erk1/2 and the phosphatidylinositol 3-kinase substrate Akt. Treatment of cardiomyocytes with inhibitors of phosphatidylinositol 3-kinase (LY294002) or Akt (Akti-1/2) abolished the protective effect of erythropoietin, whereas treatment with MAPK kinase (MEK1) inhibitor U0126 did not. Erythropoietin also induced the phosphorylation of GSK-3beta, a downstream target of PI3K-Akt. Because phosphorylation is known to inactivate GSK-3beta, we investigated whether GSK-3beta inhibition is cardioprotective. We found that GSK-3beta inhibitors SB216763 or lithium chloride blocked doxorubicin-induced cardiomyocyte apoptosis in a manner similar to erythropoietin, suggesting that GSK-3beta inhibition is involved in erythropoietin-mediated cardioprotection. Erythropoietin may serve as a novel cardioprotective agent against anthracycline-induced cardiotoxicity.
Figures




Similar articles
-
Erythropoietin protects against doxorubicin-induced cardiomyopathy via a phosphatidylinositol 3-kinase-dependent pathway.J Pharmacol Exp Ther. 2008 Jan;324(1):160-9. doi: 10.1124/jpet.107.125773. Epub 2007 Oct 10. J Pharmacol Exp Ther. 2008. PMID: 17928571
-
Over-expression of calpastatin aggravates cardiotoxicity induced by doxorubicin.Cardiovasc Res. 2013 Jun 1;98(3):381-90. doi: 10.1093/cvr/cvt048. Epub 2013 Mar 1. Cardiovasc Res. 2013. PMID: 23455548
-
Testosterone Antagonizes Doxorubicin-Induced Senescence of Cardiomyocytes.J Am Heart Assoc. 2016 Jan 8;5(1):e002383. doi: 10.1161/JAHA.115.002383. J Am Heart Assoc. 2016. PMID: 26746999 Free PMC article.
-
[Molecular mechanisms of the cardiotoxic action of anthracycline antibiotics and statin-induced cytoprotective reactions of cardiomyocytes].Biomed Khim. 2020 Sep;66(5):357-371. doi: 10.18097/PBMC20206605357. Biomed Khim. 2020. PMID: 33140729 Review. Russian.
-
Anthracycline-derived chemotherapeutics in apoptosis and free radical cytotoxicity (Review).Int J Mol Med. 1998 Feb;1(2):491-4. doi: 10.3892/ijmm.1.2.491. Int J Mol Med. 1998. PMID: 9852255 Review.
Cited by
-
Cardiac toxicity: old and new issues in anti-cancer drugs.Clin Transl Oncol. 2008 Jan;10(1):35-46. doi: 10.1007/s12094-008-0150-8. Clin Transl Oncol. 2008. PMID: 18208791 Review.
-
Cardiac antiapoptotic and proproliferative effect of recombinant human erythropoietin in a moderate stage of chronic renal failure in the rat.J Pharm Bioallied Sci. 2012 Jan;4(1):76-83. doi: 10.4103/0975-7406.92743. J Pharm Bioallied Sci. 2012. PMID: 22368404 Free PMC article.
-
Chemotherapy-related Cardiomyopathy.Eur Cardiol. 2015 Jul;10(1):19-24. doi: 10.15420/ecr.2015.10.01.19. Eur Cardiol. 2015. PMID: 30310418 Free PMC article. Review.
-
Endogenous erythropoietin signaling facilitates skeletal muscle repair and recovery following pharmacologically induced damage.FASEB J. 2012 Jul;26(7):2847-58. doi: 10.1096/fj.11-196618. Epub 2012 Apr 9. FASEB J. 2012. PMID: 22490927 Free PMC article.
-
Pharmacological management of cardiorenal syndromes.Int J Nephrol. 2011;2011:630809. doi: 10.4061/2011/630809. Epub 2011 May 26. Int J Nephrol. 2011. PMID: 21660311 Free PMC article.
References
-
- Dorr RT. Cytoprotective agents for anthracyclines. Semin Oncol. 1996;23:23–34. - PubMed
-
- Arola OJ, Saraste A, Pulkki K, Kallajoki M, Parvinen M, Voipio-Pulkki LM. Acute doxorubicin cardiotoxicity involves cardiomyocyte apoptosis. Cancer Res. 2000;60:1789–1792. - PubMed
-
- Yamaoka M, Yamaguchi S, Suzuki T, Okuyama M, Nitobe J, Nakamura N, Mitsui Y, Tomoike H. Apoptosis in rat cardiac myocytes induced by Fas ligand: priming for Fas-mediated apoptosis with doxorubicin. J Mol Cell Cardiol. 2000;32:881–889. - PubMed
-
- Kotamraju S, Konorev EA, Joseph J, Kalyanaraman B. Doxorubicin-induced apoptosis in endothelial cells and cardiomyocytes is ameliorated by nitrone spin traps and ebselen. Role of reactive oxygen and nitrogen species. J Biol Chem. 2000;275:33585–33592. - PubMed
-
- Wu H, Lee SH, Gao J, Liu X, Iruela-Arispe ML. Inactivation of erythropoietin leads to defects in cardiac morphogenesis. Development. 1999;126:3597–3605. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

