NHERF links the N-cadherin/catenin complex to the platelet-derived growth factor receptor to modulate the actin cytoskeleton and regulate cell motility
- PMID: 17229887
- PMCID: PMC1838972
- DOI: 10.1091/mbc.e06-10-0960
NHERF links the N-cadherin/catenin complex to the platelet-derived growth factor receptor to modulate the actin cytoskeleton and regulate cell motility
Abstract
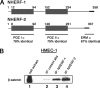
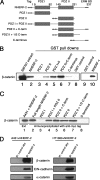
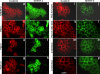
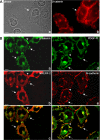
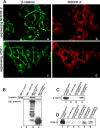
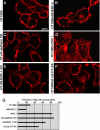
Using phage display, we identified Na+/H+ exchanger regulatory factor (NHERF)-2 as a novel binding partner for the cadherin-associated protein, beta-catenin. We showed that the second of two PSD-95/Dlg/ZO-1 (PDZ) domains of NHERF interacts with a PDZ-binding motif at the very carboxy terminus of beta-catenin. N-cadherin expression has been shown to induce motility in a number of cell types. The first PDZ domain of NHERF is known to bind platelet-derived growth factor-receptor beta (PDGF-Rbeta), and the interaction of PDGF-Rbeta with NHERF leads to enhanced cell spreading and motility. Here we show that beta-catenin and N-cadherin are in a complex with NHERF and PDGF-Rbeta at membrane ruffles in the highly invasive fibrosarcoma cell line HT1080. Using a stable short hairpin RNA system, we showed that HT1080 cells knocked down for either N-cadherin or NHERF had impaired ability to migrate into the wounded area in a scratch assay, similar to cells treated with a PDGF-R kinase inhibitor. Cells expressing a mutant NHERF that is unable to associate with beta-catenin had increased stress fibers, reduced lamellipodia, and impaired cell migration. Using HeLa cells, which express little to no PDGF-R, we introduced PDGF-Rbeta and showed that it coimmunoprecipitates with N-cadherin and that PDGF-dependent cell migration was reduced in these cells when we knocked-down expression of N-cadherin or NHERF. These studies implicate N-cadherin and beta-catenin in cell migration via PDGF-R-mediated signaling through the scaffolding molecule NHERF.
Figures




Similar articles
-
A NHERF binding site links the betaPDGFR to the cytoskeleton and regulates cell spreading and migration.J Cell Sci. 2004 Jun 15;117(Pt 14):2951-61. doi: 10.1242/jcs.01156. Epub 2004 May 25. J Cell Sci. 2004. PMID: 15161943
-
Platelet-derived growth factor receptor association with Na(+)/H(+) exchanger regulatory factor potentiates receptor activity.Mol Cell Biol. 2000 Nov;20(22):8352-63. doi: 10.1128/MCB.20.22.8352-8363.2000. Mol Cell Biol. 2000. PMID: 11046132 Free PMC article.
-
Ligand-induced recruitment of Na+/H+-exchanger regulatory factor to the PDGF (platelet-derived growth factor) receptor regulates actin cytoskeleton reorganization by PDGF.Biochem J. 2003 Dec 1;376(Pt 2):505-10. doi: 10.1042/BJ20030385. Biochem J. 2003. PMID: 12967325 Free PMC article.
-
Role of NHERF and scaffolding proteins in proximal tubule transport.Urol Res. 2010 Aug;38(4):257-62. doi: 10.1007/s00240-010-0294-1. Epub 2010 Jul 15. Urol Res. 2010. PMID: 20632170 Review.
-
Assembly of signaling complexes by the sodium-hydrogen exchanger regulatory factor family of PDZ-containing proteins.Curr Opin Nephrol Hypertens. 1999 Sep;8(5):603-8. doi: 10.1097/00041552-199909000-00012. Curr Opin Nephrol Hypertens. 1999. PMID: 10541224 Review.
Cited by
-
Increased Mesenchymal Stem Cell Functionalization in Three-Dimensional Manufacturing Settings for Enhanced Therapeutic Applications.Front Bioeng Biotechnol. 2021 Feb 12;9:621748. doi: 10.3389/fbioe.2021.621748. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 33644016 Free PMC article. Review.
-
Merlin-deficient human tumors show loss of contact inhibition and activation of Wnt/β-catenin signaling linked to the PDGFR/Src and Rac/PAK pathways.Neoplasia. 2011 Dec;13(12):1101-12. doi: 10.1593/neo.111060. Neoplasia. 2011. PMID: 22247700 Free PMC article.
-
Epithelial-mesenchymal transition in oral squamous cell carcinoma.ISRN Oncol. 2012;2012:681469. doi: 10.5402/2012/681469. Epub 2012 Mar 29. ISRN Oncol. 2012. PMID: 22548191 Free PMC article.
-
Nf2/Merlin: a coordinator of receptor signalling and intercellular contact.Br J Cancer. 2008 Jan 29;98(2):256-62. doi: 10.1038/sj.bjc.6604002. Epub 2007 Oct 30. Br J Cancer. 2008. PMID: 17971776 Free PMC article. Review.
-
Regulation of G protein-coupled receptor function by Na+/H+ exchange regulatory factors.Pharmacol Rev. 2011 Dec;63(4):882-900. doi: 10.1124/pr.110.004176. Epub 2011 Aug 26. Pharmacol Rev. 2011. PMID: 21873413 Free PMC article. Review.
References
-
- Ades E. W., Candal F. J., Swerlick R. A., George V. G., Summers S., Bosse D. C., Lawley T. J. HMEC-1, establishment of an immortalized human microvascular endothelial cell line. J. Invest. Dermatol. 1992;99:683–690. - PubMed
-
- Bienz M. beta-Catenin: a pivot between cell adhesion and Wnt signalling. Curr. Biol. 2005;15:R64–R67. - PubMed
-
- Brembeck F. H., Rosario M., Birchmeier W. Balancing cell adhesion and Wnt signaling, the key role of beta-catenin. Curr. Opin. Genet. Dev. 2006;16:51–59. - PubMed
-
- Demoulin J. B., Seo J. K., Ekman S., Grapengiesser E., Hellman U., Ronnstrand L., Heldin C. H. Ligand-induced recruitment of Na+/H+-exchanger regulatory factor to the PDGF (platelet-derived growth factor) receptor regulates actin cytoskeleton reorganization by PDGF. Biochem. J. 2003;376:505–510. - PMC - PubMed
-
- Dobrosotskaya I. Y., James G. L. MAGI-1 interacts with beta-catenin and is associated with cell-cell adhesion structures. Biochem. Biophys. Res. Commun. 2000;270:903–909. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

