Serum response factor and myocardin mediate arterial hypercontractility and cerebral blood flow dysregulation in Alzheimer's phenotype
- PMID: 17215356
- PMCID: PMC1783398
- DOI: 10.1073/pnas.0608251104
Serum response factor and myocardin mediate arterial hypercontractility and cerebral blood flow dysregulation in Alzheimer's phenotype
Abstract
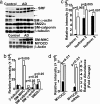
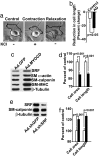
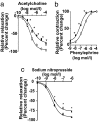
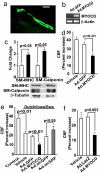
Cerebral angiopathy contributes to cognitive decline and dementia in Alzheimer's disease (AD) through cerebral blood flow (CBF) reductions and dysregulation. We report vascular smooth muscle cells (VSMC) in small pial and intracerebral arteries, which are critical for CBF regulation, express in AD high levels of serum response factor (SRF) and myocardin (MYOCD), two interacting transcription factors that orchestrate a VSMC-differentiated phenotype. Consistent with this finding, AD VSMC overexpressed several SRF-MYOCD-regulated contractile proteins and exhibited a hypercontractile phenotype. MYOCD overexpression in control human cerebral VSMC induced an AD-like hypercontractile phenotype and diminished both endothelial-dependent and -independent relaxation in the mouse aorta ex vivo. In contrast, silencing SRF normalized contractile protein content and reversed a hypercontractile phenotype in AD VSMC. MYOCD in vivo gene transfer to mouse pial arteries increased contractile protein content and diminished CBF responses produced by brain activation in wild-type mice and in two AD models, the Dutch/Iowa/Swedish triple mutant human amyloid beta-peptide (Abeta)-precursor protein (APP)- expressing mice and APPsw(+/-) mice. Silencing Srf had the opposite effect. Expression of SRF did not change in VSMC subjected to Alzheimer's neurotoxin, Abeta. Thus, SRF-MYOCD overexpression in small cerebral arteries appears to initiate independently of Abeta a pathogenic pathway mediating arterial hypercontractility and CBF dysregulation, which are associated with Alzheimer's dementia.
Conflict of interest statement
Conflict of interest: B.V.Z. is the scientific founder of Socratech L.L.C., a startup biotechnology company with a mission to develop neuroprotective strategies in the aging brain and for brain disorders such as stroke, Alzheimer's disease, and other neurodegenerative disorders. All other authors declare no conflict of interest.
Figures
Similar articles
-
SRF and myocardin regulate LRP-mediated amyloid-beta clearance in brain vascular cells.Nat Cell Biol. 2009 Feb;11(2):143-53. doi: 10.1038/ncb1819. Epub 2008 Dec 21. Nat Cell Biol. 2009. PMID: 19098903 Free PMC article.
-
Selective expression of TSPAN2 in vascular smooth muscle is independently regulated by TGF-β1/SMAD and myocardin/serum response factor.FASEB J. 2017 Jun;31(6):2576-2591. doi: 10.1096/fj.201601021R. Epub 2017 Mar 3. FASEB J. 2017. PMID: 28258189 Free PMC article.
-
MYOSLID Is a Novel Serum Response Factor-Dependent Long Noncoding RNA That Amplifies the Vascular Smooth Muscle Differentiation Program.Arterioscler Thromb Vasc Biol. 2016 Oct;36(10):2088-99. doi: 10.1161/ATVBAHA.116.307879. Epub 2016 Jul 21. Arterioscler Thromb Vasc Biol. 2016. PMID: 27444199 Free PMC article.
-
Neurovascular mechanisms and blood-brain barrier disorder in Alzheimer's disease.Acta Neuropathol. 2009 Jul;118(1):103-13. doi: 10.1007/s00401-009-0522-3. Epub 2009 Mar 25. Acta Neuropathol. 2009. PMID: 19319544 Free PMC article. Review.
-
Cerebral amyloid angiopathy: pathogenesis and effects on the ageing and Alzheimer brain.Neurol Res. 2003 Sep;25(6):611-6. doi: 10.1179/016164103101202057. Neurol Res. 2003. PMID: 14503015 Review.
Cited by
-
Vascular smooth muscle cell dysfunction in neurodegeneration.Front Neurosci. 2022 Nov 10;16:1010164. doi: 10.3389/fnins.2022.1010164. eCollection 2022. Front Neurosci. 2022. PMID: 36440263 Free PMC article. Review.
-
Impaired vascular-mediated clearance of brain amyloid beta in Alzheimer's disease: the role, regulation and restoration of LRP1.Front Aging Neurosci. 2015 Jul 15;7:136. doi: 10.3389/fnagi.2015.00136. eCollection 2015. Front Aging Neurosci. 2015. PMID: 26236233 Free PMC article. Review.
-
Multiple Factors Involved in the Pathogenesis of White Matter Lesions.Biomed Res Int. 2017;2017:9372050. doi: 10.1155/2017/9372050. Epub 2017 Feb 21. Biomed Res Int. 2017. PMID: 28316994 Free PMC article. Review.
-
Atorvastatin suppresses vascular hypersensitivity and remodeling induced by transient adventitial administration of lipopolysaccharide in rats.Ann Transl Med. 2019 Aug;7(16):386. doi: 10.21037/atm.2019.07.50. Ann Transl Med. 2019. PMID: 31555700 Free PMC article.
-
Myocardin marks the earliest cardiac gene expression and plays an important role in heart development.Anat Rec (Hoboken). 2008 Oct;291(10):1200-11. doi: 10.1002/ar.20756. Anat Rec (Hoboken). 2008. PMID: 18780304 Free PMC article.
References
-
- Zlokovic BV. Trends Neurosci. 2005;28:202–208. - PubMed
-
- Iadecola C. Nat Neurosci Rev. 2004;5:347–360. - PubMed
-
- Greenberg SM, Gurol ME, Rosand J, Smith EE. Stroke. 2004;35:2616–2619. - PubMed
-
- O'Brien JT, Erkinjuntti T, Reisberg B, Roman G, Sawada T, Pantoni L, Bowler JV, Ballard C, DeCarli C, Gorelick PB, et al. Lancet Neurol. 2003;2:89–98. - PubMed
-
- Casserly I, Topol E. Lancet. 2004;353:1139–1146. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

