Fibrosis and cancer: do myofibroblasts come also from epithelial cells via EMT?
- PMID: 17211838
- PMCID: PMC2838476
- DOI: 10.1002/jcb.21186
Fibrosis and cancer: do myofibroblasts come also from epithelial cells via EMT?
Abstract
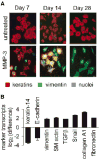
Myofibroblasts produce and modify the extracellular matrix (ECM), secrete angiogenic and pro-inflammatory factors, and stimulate epithelial cell proliferation and invasion. Myofibroblasts are normally induced transiently during wound healing, but inappropriate induction of myofibroblasts causes organ fibrosis, which greatly enhances the risk of subsequent cancer development. As myofibroblasts are also found in the reactive tumor stroma, the processes involved in their development and activation are an area of active investigation. Emerging evidence suggests that a major source of fibrosis- and tumor-associated myofibroblasts is through transdifferentiation from non-malignant epithelial or epithelial-derived carcinoma cells through epithelial-mesenchymal transition (EMT). This review will focus on the role of EMT in fibrosis, considered in the context of recent studies showing that exposure of epithelial cells to matrix metalloproteinases (MMPs) can lead to increased levels of cellular reactive oxygen species (ROS) that stimulate transdifferentiation to myofibroblast-like cells. As deregulated MMP expression and increased cellular ROS are characteristic of both fibrosis and malignancy, these studies suggest that increased MMP expression may stimulate fibrosis, tumorigenesis, and tumor progression by inducing a specialized EMT in which epithelial cells transdifferentiate into activated myofibroblasts. This connection provides a new perspective on the development of the fibrosis and tumor microenvironments.
Figures


Similar articles
-
Matrix metalloproteinase-induced epithelial-mesenchymal transition: tumor progression at Snail's pace.Int J Biochem Cell Biol. 2007;39(6):1082-8. doi: 10.1016/j.biocel.2007.03.002. Epub 2007 Mar 12. Int J Biochem Cell Biol. 2007. PMID: 17416542 Review.
-
Matrix metalloproteinase-induced malignancy in mammary epithelial cells.Cells Tissues Organs. 2007;185(1-3):104-10. doi: 10.1159/000101310. Cells Tissues Organs. 2007. PMID: 17587815 Review.
-
Matrix metalloproteinase-induced fibrosis and malignancy in breast and lung.Proc Am Thorac Soc. 2008 Apr 15;5(3):316-22. doi: 10.1513/pats.200711-166DR. Proc Am Thorac Soc. 2008. PMID: 18403326
-
[Epithelial-mesenchymal transition in cancer progression].Postepy Biochem. 2009;55(2):121-8. Postepy Biochem. 2009. PMID: 19824467 Review. Polish.
-
The role of epithelial-mesenchymal transition in cancer pathology.Pathology. 2007 Jun;39(3):305-18. doi: 10.1080/00313020701329914. Pathology. 2007. PMID: 17558857 Review.
Cited by
-
Cell-cell and cell-matrix dynamics in intraperitoneal cancer metastasis.Cancer Metastasis Rev. 2012 Jun;31(1-2):397-414. doi: 10.1007/s10555-012-9351-2. Cancer Metastasis Rev. 2012. PMID: 22527451 Free PMC article. Review.
-
The NRF2-heme oxygenase-1 system modulates cyclosporin A-induced epithelial-mesenchymal transition and renal fibrosis.Free Radic Biol Med. 2010 Apr 15;48(8):1051-63. doi: 10.1016/j.freeradbiomed.2010.01.021. Epub 2010 Jan 22. Free Radic Biol Med. 2010. PMID: 20096777 Free PMC article.
-
Extracellular matrix stiffness-The central cue for skin fibrosis.Front Mol Biosci. 2023 Mar 8;10:1132353. doi: 10.3389/fmolb.2023.1132353. eCollection 2023. Front Mol Biosci. 2023. PMID: 36968277 Free PMC article. Review.
-
Pyruvate Kinase M2: a Metabolic Bug in Re-Wiring the Tumor Microenvironment.Cancer Microenviron. 2019 Dec;12(2-3):149-167. doi: 10.1007/s12307-019-00226-0. Epub 2019 Jun 10. Cancer Microenviron. 2019. PMID: 31183810 Free PMC article. Review.
-
High levels of microRNA-21 in the stroma of colorectal cancers predict short disease-free survival in stage II colon cancer patients.Clin Exp Metastasis. 2011 Jan;28(1):27-38. doi: 10.1007/s10585-010-9355-7. Epub 2010 Oct 31. Clin Exp Metastasis. 2011. PMID: 21069438 Free PMC article.
References
-
- Artinian V, Kvale PA. Cancer and interstitial lung disease. Curr Opin Pulm Med. 2004;10:425–434. - PubMed
-
- Bartow SA, Pathak DR, Mettler FA. Radiographic microcalcification and parenchymal patterns as indicators of histologic “high-risk” benign breast disease. Cancer. 1990;66:1721–1725. - PubMed
-
- Bissell DM. Chronic liver injury, TGF-beta, and cancer. Exp Mol Med. 2001;33:179–190. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

