Mechanisms underlying the resistance to diet-induced obesity in germ-free mice
- PMID: 17210919
- PMCID: PMC1764762
- DOI: 10.1073/pnas.0605374104
Mechanisms underlying the resistance to diet-induced obesity in germ-free mice
Abstract
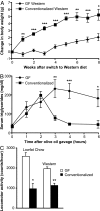
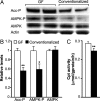
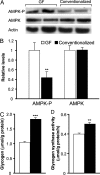
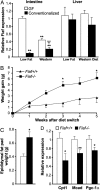
The trillions of microbes that colonize our adult intestines function collectively as a metabolic organ that communicates with, and complements, our own human metabolic apparatus. Given the worldwide epidemic in obesity, there is interest in how interactions between human and microbial metabolomes may affect our energy balance. Here we report that, in contrast to mice with a gut microbiota, germ-free (GF) animals are protected against the obesity that develops after consuming a Western-style, high-fat, sugar-rich diet. Their persistently lean phenotype is associated with increased skeletal muscle and liver levels of phosphorylated AMP-activated protein kinase (AMPK) and its downstream targets involved in fatty acid oxidation (acetylCoA carboxylase; carnitine-palmitoyltransferase). Moreover, GF knockout mice lacking fasting-induced adipose factor (Fiaf), a circulating lipoprotein lipase inhibitor whose expression is normally selectively suppressed in the gut epithelium by the microbiota, are not protected from diet-induced obesity. Although GF Fiaf-/- animals exhibit similar levels of phosphorylated AMPK as their wild-type littermates in liver and gastrocnemius muscle, they have reduced expression of genes encoding the peroxisomal proliferator-activated receptor coactivator (Pgc-1alpha) and enzymes involved in fatty acid oxidation. Thus, GF animals are protected from diet-induced obesity by two complementary but independent mechanisms that result in increased fatty acid metabolism: (i) elevated levels of Fiaf, which induces Pgc-1alpha; and (ii) increased AMPK activity. Together, these findings support the notion that the gut microbiota can influence both sides of the energy balance equation, and underscore the importance of considering our metabolome in a supraorganismal context.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Effects of alpha-AMPK knockout on exercise-induced gene activation in mouse skeletal muscle.FASEB J. 2005 Jul;19(9):1146-8. doi: 10.1096/fj.04-3144fje. Epub 2005 May 5. FASEB J. 2005. PMID: 15878932
-
AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha.Proc Natl Acad Sci U S A. 2007 Jul 17;104(29):12017-22. doi: 10.1073/pnas.0705070104. Epub 2007 Jul 3. Proc Natl Acad Sci U S A. 2007. PMID: 17609368 Free PMC article.
-
Enhanced polyamine catabolism alters homeostatic control of white adipose tissue mass, energy expenditure, and glucose metabolism.Mol Cell Biol. 2007 Jul;27(13):4953-67. doi: 10.1128/MCB.02034-06. Epub 2007 May 7. Mol Cell Biol. 2007. PMID: 17485446 Free PMC article.
-
Stearoyl-CoA desaturase--a new player in skeletal muscle metabolism regulation.J Physiol Pharmacol. 2006 Nov;57 Suppl 10:31-42. J Physiol Pharmacol. 2006. PMID: 17242489 Review.
-
Regulation of energy metabolism by long-chain fatty acids.Prog Lipid Res. 2014 Jan;53:124-44. doi: 10.1016/j.plipres.2013.12.001. Epub 2013 Dec 18. Prog Lipid Res. 2014. PMID: 24362249 Review.
Cited by
-
Probiotics, prebiotics, synbiotics and other microbiome-based innovative therapeutics to mitigate obesity and enhance longevity via the gut-brain axis.Microbiome Res Rep. 2024 May 17;3(3):29. doi: 10.20517/mrr.2024.05. eCollection 2024. Microbiome Res Rep. 2024. PMID: 39421246 Free PMC article. Review.
-
Gut microbiome therapy: fecal microbiota transplantation vs live biotherapeutic products.Gut Microbes. 2024 Jan-Dec;16(1):2412376. doi: 10.1080/19490976.2024.2412376. Epub 2024 Oct 8. Gut Microbes. 2024. PMID: 39377231 Free PMC article. Review.
-
Delayed Gut Colonization Changes Future Insulin Resistance and Hepatic Gene Expression but Not Adiposity in Obese Mice.J Obes. 2024 Sep 25;2024:5846674. doi: 10.1155/2024/5846674. eCollection 2024. J Obes. 2024. PMID: 39360185 Free PMC article.
-
Dietary and metabolic effects on intestinal stem cells in health and disease.Nat Rev Gastroenterol Hepatol. 2024 Oct 2. doi: 10.1038/s41575-024-00980-7. Online ahead of print. Nat Rev Gastroenterol Hepatol. 2024. PMID: 39358589 Review.
-
A systematic framework for understanding the microbiome in human health and disease: from basic principles to clinical translation.Signal Transduct Target Ther. 2024 Sep 23;9(1):237. doi: 10.1038/s41392-024-01946-6. Signal Transduct Target Ther. 2024. PMID: 39307902 Free PMC article. Review.
References
-
- Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Science. 2005;307:1915–1920. - PubMed
-
- Wolin M. Science. 1981;213:1463–1468. - PubMed
-
- Merkel M, Eckel RH, Goldberg IJ. J Lipid Res. 2002;43:1997–2006. - PubMed
-
- Preiss-Landl K, Zimmermann R, Hammerle G, Zechner R. Curr Opin Lipidol. 2002;13:471–481. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

