The effects of second-hand smoke on biological processes important in atherogenesis
- PMID: 17210084
- PMCID: PMC1774583
- DOI: 10.1186/1471-2261-7-1
The effects of second-hand smoke on biological processes important in atherogenesis
Abstract
Background: Atherosclerosis is the leading cause of death in western societies and cigarette smoke is among the factors that strongly contribute to the development of this disease. The early events in atherogenesis are stimulated on the one hand by cytokines that chemoattract leukocytes and on the other hand by decrease in circulating molecules that protect endothelial cells (ECs) from injury. Here we focus our studies on the effects of "second-hand" smoke on atherogenesis.
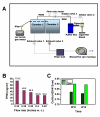
Methods: To perform these studies, a smoking system that closely simulates exposure of humans to second-hand smoke was developed and a mouse model system transgenic for human apoB100 was used. These mice have moderate lipid levels that closely mimic human conditions that lead to atherosclerotic plaque formation.
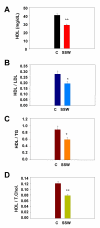
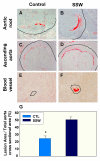
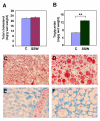
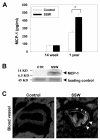
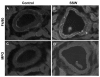
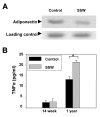
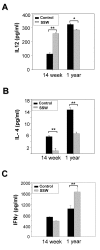
Results: "Second-hand" cigarette smoke decreases plasma high density lipoprotein levels in the blood and also decreases the ratios between high density lipoprotein and low density lipoprotein, high density lipoprotein and triglyceride, and high density lipoprotein and total cholesterol. This change in lipid profiles causes not only more lipid accumulation in the aorta but also lipid deposition in many of the smaller vessels of the heart and in hepatocytes. In addition, mice exposed to smoke have increased levels of Monocyte Chemoattractant Protein-1 in circulation and in the heart/aorta tissue, have increased macrophages in the arterial walls, and have decreased levels of adiponectin, an EC-protective protein. Also, cytokine arrays revealed that mice exposed to smoke do not undergo the switch from the pro-inflammatory cytokine profile (that develops when the mice are initially exposed to second-hand smoke) to the adaptive response. Furthermore, triglyceride levels increase significantly in the liver of smoke-exposed mice.
Conclusion: Long-term exposure to "second-hand" smoke creates a state of permanent inflammation and an imbalance in the lipid profile that leads to lipid accumulation in the liver and in the blood vessels of the heart and aorta. The former potentially can lead to non-alcoholic fatty liver disease and the latter to heart attacks.
Figures

Similar articles
-
P55 tumour necrosis factor receptor in bone marrow-derived cells promotes atherosclerosis development in low-density lipoprotein receptor knock-out mice.Cardiovasc Res. 2008 Nov 1;80(2):309-18. doi: 10.1093/cvr/cvn193. Epub 2008 Jul 15. Cardiovasc Res. 2008. PMID: 18628255
-
Molecular mechanisms of felodipine suppressing atherosclerosis in high-cholesterol-diet apolipoprotein E-knockout mice.J Cardiovasc Pharmacol. 2008 Feb;51(2):188-95. doi: 10.1097/FJC.0b013e31815f2bce. J Cardiovasc Pharmacol. 2008. PMID: 18287887
-
Human paraoxonase gene cluster transgenic overexpression represses atherogenesis and promotes atherosclerotic plaque stability in ApoE-null mice.Circ Res. 2009 May 22;104(10):1160-8. doi: 10.1161/CIRCRESAHA.108.192229. Epub 2009 Apr 9. Circ Res. 2009. PMID: 19359600
-
Lipids and atherosclerosis.Biochem Cell Biol. 2004 Feb;82(1):212-24. doi: 10.1139/o03-085. Biochem Cell Biol. 2004. PMID: 15052339 Review.
-
The inflammation: lipoprotein cycle.Atheroscler Suppl. 2005 May;6(2):15-20. doi: 10.1016/j.atherosclerosissup.2005.02.004. Atheroscler Suppl. 2005. PMID: 15823492 Review.
Cited by
-
Particulate matter and atherosclerosis: a bibliometric analysis of original research articles published in 1973-2014.BMC Public Health. 2016 Apr 19;16:348. doi: 10.1186/s12889-016-3015-z. BMC Public Health. 2016. PMID: 27093947 Free PMC article. Review.
-
Transgenerational exposure to environmental tobacco smoke.Int J Environ Res Public Health. 2014 Jul 16;11(7):7261-74. doi: 10.3390/ijerph110707261. Int J Environ Res Public Health. 2014. PMID: 25032741 Free PMC article. Review.
-
Secondhand smoking increased the possibility of hypertension with a significant time and frequency dose-response relationship.Sci Rep. 2024 Oct 23;14(1):24950. doi: 10.1038/s41598-024-76055-z. Sci Rep. 2024. PMID: 39438598 Free PMC article.
-
Environmental tobacco smoke and children's health.Korean J Pediatr. 2012 Feb;55(2):35-41. doi: 10.3345/kjp.2012.55.2.35. Epub 2012 Feb 14. Korean J Pediatr. 2012. PMID: 22375147 Free PMC article.
-
Second-hand smoke stimulates lipid accumulation in the liver by modulating AMPK and SREBP-1.J Hepatol. 2009 Sep;51(3):535-47. doi: 10.1016/j.jhep.2009.03.026. Epub 2009 May 18. J Hepatol. 2009. PMID: 19556020 Free PMC article.
References
-
- Bruce I, McNally J, Bell A. Enhanced monocyte generation of reactive oxygen species in primary systemic vasculitis. J Rheumatol. 1997;24:2364–2370. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

