Cyclin A2 regulates nuclear-envelope breakdown and the nuclear accumulation of cyclin B1
- PMID: 17208191
- PMCID: PMC1830184
- DOI: 10.1016/j.cub.2006.11.066
Cyclin A2 regulates nuclear-envelope breakdown and the nuclear accumulation of cyclin B1
Abstract
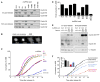
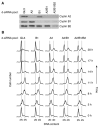
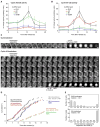
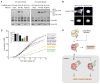
Mitosis is thought to be triggered by the activation of Cdk-cyclin complexes. Here we have used RNA interference (RNAi) to assess the roles of three mitotic cyclins, cyclins A2, B1, and B2, in the regulation of centrosome separation and nuclear-envelope breakdown (NEB) in HeLa cells. We found that the timing of NEB was affected very little by knocking down cyclins B1 and B2 alone or in combination. However, knocking down cyclin A2 markedly delayed NEB, and knocking down both cyclins A2 and B1 delayed NEB further. The timing of cyclin B1-Cdk1 activation was normal in cyclin A2 knockdown cells, and there was no delay in centrosome separation, an event apparently controlled by the activation of cytoplasmic cyclin B1-Cdk1. However, nuclear accumulation of cyclin B1-Cdk1 was markedly delayed in cyclin A2 knockdown cells. Finally, a constitutively nuclear cyclin B1, but not wild-type cyclin B1, restored normal NEB timing in cyclin A2 knockdown cells. These findings show that cyclin A2 is required for timely NEB, whereas cyclins B1 and B2 are not. Nevertheless cyclin B1 translocates to the nucleus just prior to NEB in a cyclin A2-dependent fashion and is capable of supporting NEB if rendered constitutively nuclear.
Figures
Similar articles
-
The roles of cyclin A2, B1, and B2 in early and late mitotic events.Mol Biol Cell. 2010 Sep 15;21(18):3149-61. doi: 10.1091/mbc.E10-05-0393. Epub 2010 Jul 21. Mol Biol Cell. 2010. PMID: 20660152 Free PMC article.
-
Cyclin B2 suppresses mitotic failure and DNA re-replication in human somatic cells knocked down for both cyclins B1 and B2.Oncogene. 2007 Nov 8;26(51):7175-84. doi: 10.1038/sj.onc.1210539. Epub 2007 May 28. Oncogene. 2007. PMID: 17533373
-
Cyclin B1-Cdk1 activation continues after centrosome separation to control mitotic progression.PLoS Biol. 2007 May;5(5):e123. doi: 10.1371/journal.pbio.0050123. PLoS Biol. 2007. PMID: 17472438 Free PMC article.
-
Control of mitosis by changes in the subcellular location of cyclin-B1-Cdk1 and Cdc25C.Curr Opin Cell Biol. 2000 Dec;12(6):658-65. doi: 10.1016/s0955-0674(00)00149-6. Curr Opin Cell Biol. 2000. PMID: 11063929 Review.
-
Cyclin B1 and CDK1: nuclear localization and upstream regulators.Prog Cell Cycle Res. 2003;5:335-47. Prog Cell Cycle Res. 2003. PMID: 14593728 Review.
Cited by
-
Unique Regulatory Mechanisms for the Human Embryonic Stem Cell Cycle.J Cell Physiol. 2017 Jun;232(6):1254-1257. doi: 10.1002/jcp.25567. Epub 2017 Jan 31. J Cell Physiol. 2017. PMID: 27532275 Free PMC article. Review.
-
The Apparent Requirement for Protein Synthesis during G2 Phase Is due to Checkpoint Activation.Cell Rep. 2020 Jul 14;32(2):107901. doi: 10.1016/j.celrep.2020.107901. Cell Rep. 2020. PMID: 32668239 Free PMC article.
-
Lamin b1 polymorphism influences morphology of the nuclear envelope, cell cycle progression, and risk of neural tube defects in mice.PLoS Genet. 2012;8(11):e1003059. doi: 10.1371/journal.pgen.1003059. Epub 2012 Nov 15. PLoS Genet. 2012. PMID: 23166514 Free PMC article.
-
Global gene expression profiling of human pleural mesotheliomas: identification of matrix metalloproteinase 14 (MMP-14) as potential tumour target.PLoS One. 2009 Sep 15;4(9):e7016. doi: 10.1371/journal.pone.0007016. PLoS One. 2009. PMID: 19753302 Free PMC article.
-
As a downstream target of the AKT pathway, NPTX1 inhibits proliferation and promotes apoptosis in hepatocellular carcinoma.Biosci Rep. 2019 Jun 4;39(6):BSR20181662. doi: 10.1042/BSR20181662. Print 2019 Jun 28. Biosci Rep. 2019. PMID: 31113871 Free PMC article.
References
-
- Hunt T. Cyclins and their partners: from a simple idea to complicated reality. Semin Cell Biol. 1991;2:213–222. - PubMed
-
- Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990;344:503–508. - PubMed
-
- Jackman M, Lindon C, Nigg EA, Pines J. Active cyclin B1-Cdk1 first appears on centrosomes in prophase. Nat Cell Biol. 2003;5:143–148. - PubMed
-
- Myers JW, Jones JT, Meyer T, Ferrell JE., Jr Recombinant Dicer efficiently converts large dsRNAs into siRNAs suitable for gene silencing. Nature Biotechnology. 2003;21:324–328. - PubMed
-
- Myers JW, Ferrell JE., Jr . Silencing gene expression with Dicer-generated siRNA pools. In: Carmichael GG, editor. RNA Silencing: Methods and Protocols. Vol. 309. Totowa NJ: Humana Press Inc; 2005. pp. 93–196. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

