C-type natriuretic peptide activates a non-selective cation current in acutely isolated rat cardiac fibroblasts via natriuretic peptide C receptor-mediated signalling
- PMID: 17204501
- PMCID: PMC2075416
- DOI: 10.1113/jphysiol.2006.120832
C-type natriuretic peptide activates a non-selective cation current in acutely isolated rat cardiac fibroblasts via natriuretic peptide C receptor-mediated signalling
Abstract
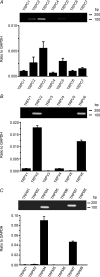
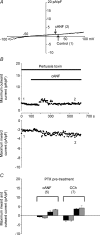
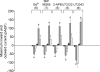
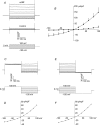
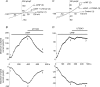
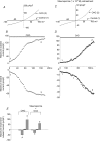
In the heart, fibroblasts play an essential role in the deposition of the extracellular matrix and they also secrete a number of hormonal factors. Although natriuretic peptides, including C-type natriuretic peptide (CNP) and brain natriuretic peptide, have antifibrotic effects on cardiac fibroblasts, the effects of CNP on fibroblast electrophysiology have not been examined. In this study, acutely isolated ventricular fibroblasts from the adult rat were used to measure the effects of CNP (2 x 10(-8) M) under whole-cell voltage-clamp conditions. CNP, as well as the natriuretic peptide C receptor (NPR-C) agonist cANF (2 x 10(-8) M), significantly increased an outwardly rectifying non-selective cation current (NSCC). This current has a reversal potential near 0 mV. Activation of this NSCC by cANF was abolished by pre-treating fibroblasts with pertussis toxin, indicating the involvement of G(i) proteins. The cANF-activated NSCC was inhibited by the compounds Gd(3+), SKF 96365 and 2-aminoethoxydiphenyl borate. Quantitative RT-PCR analysis of mRNA from rat ventricular fibroblasts revealed the expression of several transient receptor potential (TRP) channel transcripts. Additional electrophysiological analysis showed that U73122, a phospholipase C antagonist, inhibited the cANF-activated NSCC. Furthermore, the effects of CNP and cANF were mimicked by the diacylglycerol analogue 1-oleoyl-2-acetyl-sn-glycerol (OAG), independently of protein kinase C activity. These are defining characteristics of specific TRPC channels. More detailed molecular analysis confirmed the expression of full-length TRPC2, TRPC3 and TRPC5 transcripts. These data indicate that CNP, acting via the NPR-C receptor, activates a NSCC that is at least partially carried by TRPC channels in cardiac fibroblasts.
Figures




Similar articles
-
C-type natriuretic peptide inhibits L-type Ca2+ current in rat magnocellular neurosecretory cells by activating the NPR-C receptor.J Neurophysiol. 2005 Jul;94(1):612-21. doi: 10.1152/jn.00057.2005. Epub 2005 Mar 16. J Neurophysiol. 2005. PMID: 15772242
-
C-type natriuretic peptide enhances amylase release through NPR-C receptors in the exocrine pancreas.Am J Physiol Gastrointest Liver Physiol. 2007 Nov;293(5):G987-94. doi: 10.1152/ajpgi.00268.2007. Epub 2007 Aug 16. Am J Physiol Gastrointest Liver Physiol. 2007. PMID: 17702953
-
Effects of C-type natriuretic peptide on ionic currents in mouse sinoatrial node: a role for the NPR-C receptor.Am J Physiol Heart Circ Physiol. 2004 May;286(5):H1970-7. doi: 10.1152/ajpheart.00893.2003. Epub 2004 Jan 2. Am J Physiol Heart Circ Physiol. 2004. PMID: 14704228
-
Natriuretic peptide C receptor signalling in the heart and vasculature.J Physiol. 2008 Jan 15;586(2):353-66. doi: 10.1113/jphysiol.2007.144253. Epub 2007 Nov 15. J Physiol. 2008. PMID: 18006579 Free PMC article. Review.
-
Natriuretic peptide receptor-C signaling and regulation.Peptides. 2005 Jun;26(6):1044-59. doi: 10.1016/j.peptides.2004.09.023. Epub 2005 Apr 8. Peptides. 2005. PMID: 15911072 Review.
Cited by
-
Transient receptor potential (TRP) channels and cardiac fibrosis.Curr Top Med Chem. 2013;13(3):270-82. doi: 10.2174/1568026611313030005. Curr Top Med Chem. 2013. PMID: 23432060 Free PMC article. Review.
-
TRPC Channels: Dysregulation and Ca2+ Mishandling in Ischemic Heart Disease.Cells. 2020 Jan 10;9(1):173. doi: 10.3390/cells9010173. Cells. 2020. PMID: 31936700 Free PMC article. Review.
-
Impaired sinoatrial node function and increased susceptibility to atrial fibrillation in mice lacking natriuretic peptide receptor C.J Physiol. 2015 Mar 1;593(5):1127-46. doi: 10.1113/jphysiol.2014.283135. Epub 2015 Jan 12. J Physiol. 2015. PMID: 25641115 Free PMC article.
-
The Soft- and Hard-Heartedness of Cardiac Fibroblasts: Mechanotransduction Signaling Pathways in Fibrosis of the Heart.J Clin Med. 2017 May 19;6(5):53. doi: 10.3390/jcm6050053. J Clin Med. 2017. PMID: 28534817 Free PMC article. Review.
-
A review of the literature on cardiac electrical activity between fibroblasts and myocytes.Prog Biophys Mol Biol. 2016 Jan;120(1-3):128-33. doi: 10.1016/j.pbiomolbio.2015.12.006. Epub 2015 Dec 20. Prog Biophys Mol Biol. 2016. PMID: 26713556 Free PMC article. Review.
References
-
- Allessie M, Schotten U, Verheule S, Harks E. Gene therapy for repair of cardiac fibrosis: a long way to Tipperary. Circulation. 2005;111:391–393. - PubMed
-
- Anand-Srivastava MB, Sairam MR, Cantin M. Ring-deleted analogs of atrial natriuretic factor inhibit adenylate cyclase/cAMP system. Possible coupling of clearance atrial natriuretic factor receptors to adenylate cyclase/cAMP signal transduction system. J Biol Chem. 1990;265:8566–8572. - PubMed
-
- Anand-Srivastava MB, Sehl PD, Lowe DG. Cytoplasmic domain of natriuretic peptide receptor-C inhibits adenylyl cyclase. Involvement of a pertussis toxin-sensitive G protein. J Biol Chem. 1996;271:19324–19329. - PubMed
-
- Bandyopadhyay BC, Swaim WD, Liu X, Redman RS, Patterson RL, Ambudkar IS. Apical localization of a functional TRPC3/TRPC6-Ca2+ signaling complex in polarized epithelial cells. Role in apical Ca2+ influx. J Biol Chem. 2005;280:12908–12916. - PubMed
-
- Barr AJ, Ali H, Haribabu B, Snyderman R, Smrcka AV. Identification of a region at the N-terminus of phospholipase Cβ3 that interacts with G protein βγ subunits. Biochemistry. 2000;39:1800–1806. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

